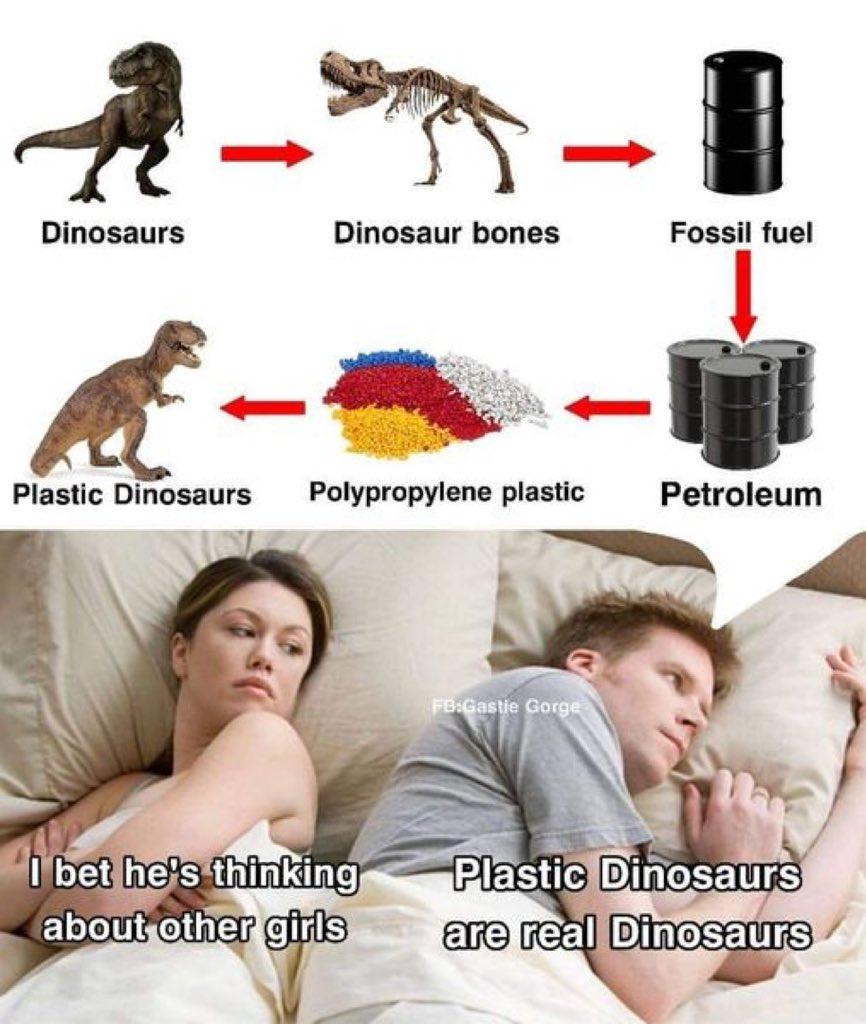
Chemistry nerds are straight up savage with this one! Pi bonds can't rotate because they're formed by side-by-side p orbitals with that electron density above and below the molecular plane. Try to rotate? Those orbitals lose overlap and the bond breaks! Meanwhile, sigma bonds are out here flexing with their free rotation abilities. It's basically the molecular equivalent of "stay in your lane" 😂
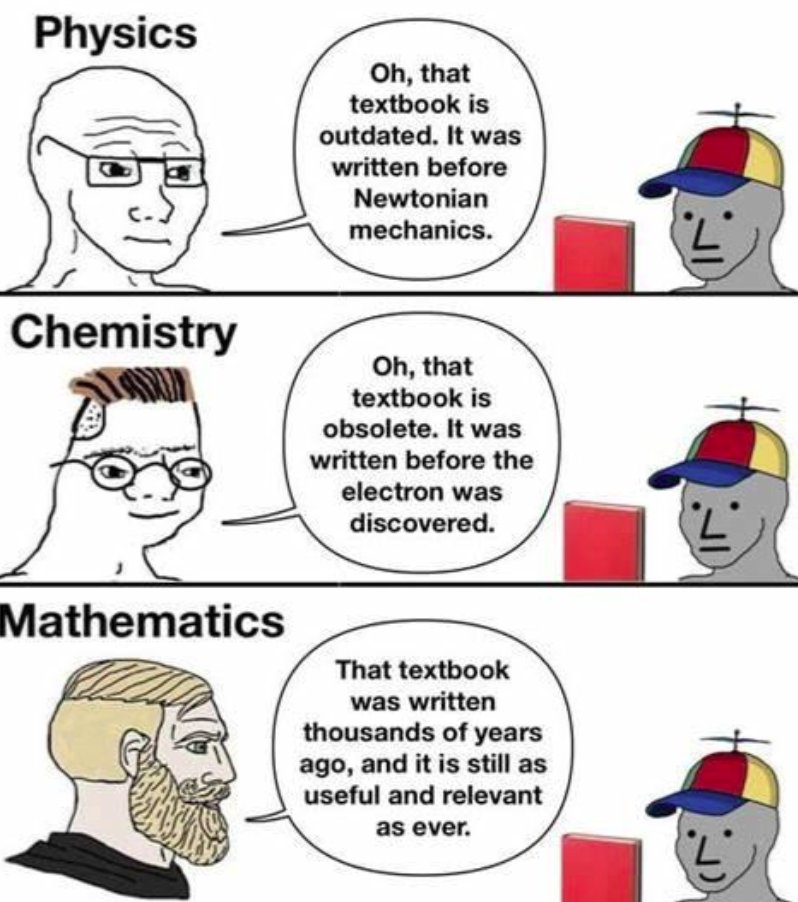
Sorry, You're Not A Sigma Bond

chemistry-memes, molecular-bonds-memes, pi-bonds-memes, sigma-bonds-memes, orbitals-memes | ScienceHumor.io
More Like This
Name Reactions: The Chemist's Eternal Nightmare
9 months ago
15.7K views
0 shares

Some Things Never Change: The Evolution Of Toxins
7 months ago
18.6K views
0 shares

Loading more content...

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology