HTTP 418: I'm a teapot
The server identifies as a teapot now and is on a tea break, brb
HTTP 418: I'm a teapot
The server identifies as a teapot now and is on a tea break, brb
Chemistry Memes
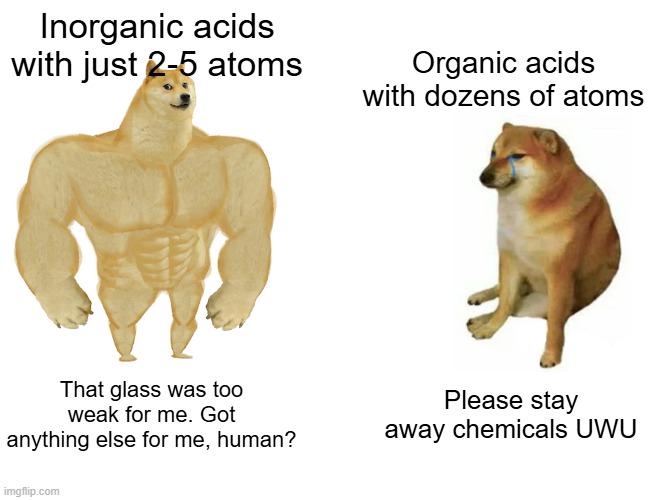
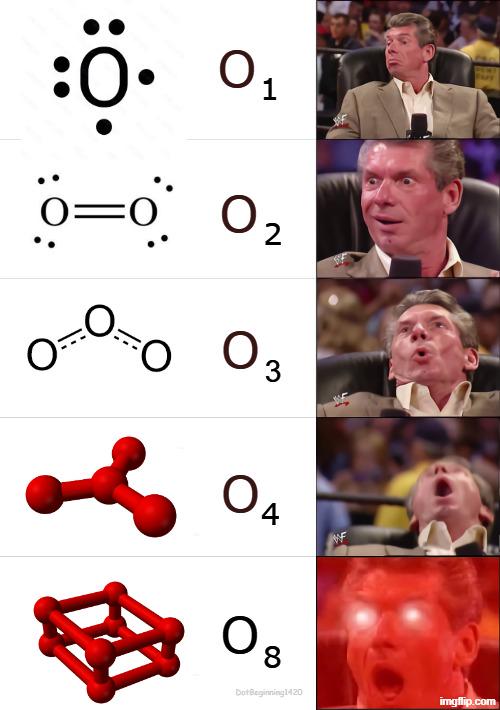
Chemistry: where "don't lick it" is an actual laboratory rule because someone, somewhere, definitely did. These memes celebrate the science of playing with substances that can change color, explode, or occasionally violate international weapons treaties. If you've ever made a terrible pun about elements, gotten way too excited about a perfect crystallization, or had to explain that no, you can't actually make Walter White's blue stuff, you'll find your periodic table pals here. From the satisfying precision of a perfectly balanced equation to the existential dread of organic synthesis, ScienceHumor.io's chemistry collection captures the beautiful chaos of a field where "flammable" and "inflammable" mean the same thing just to confuse undergrads.

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology