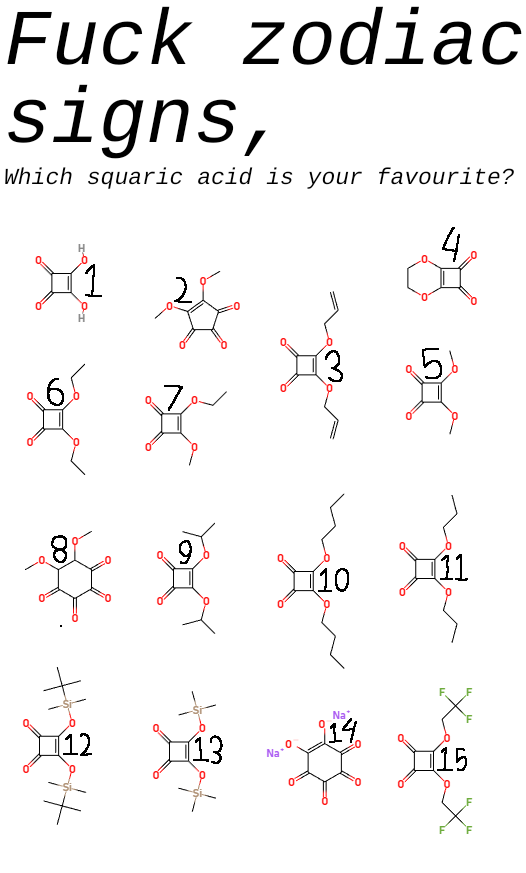
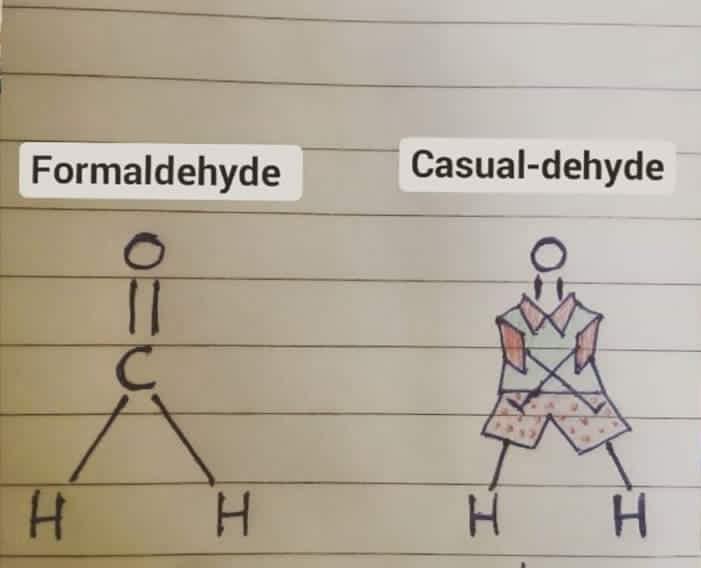
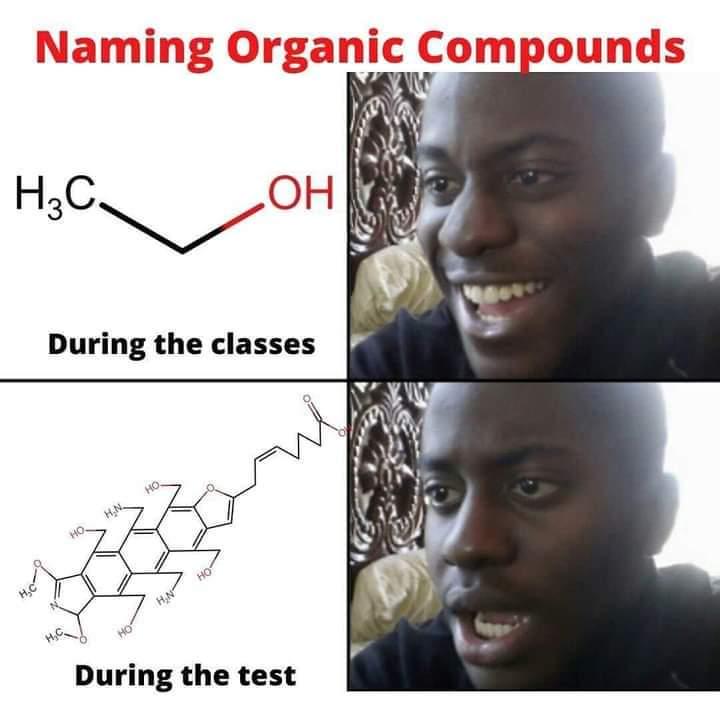
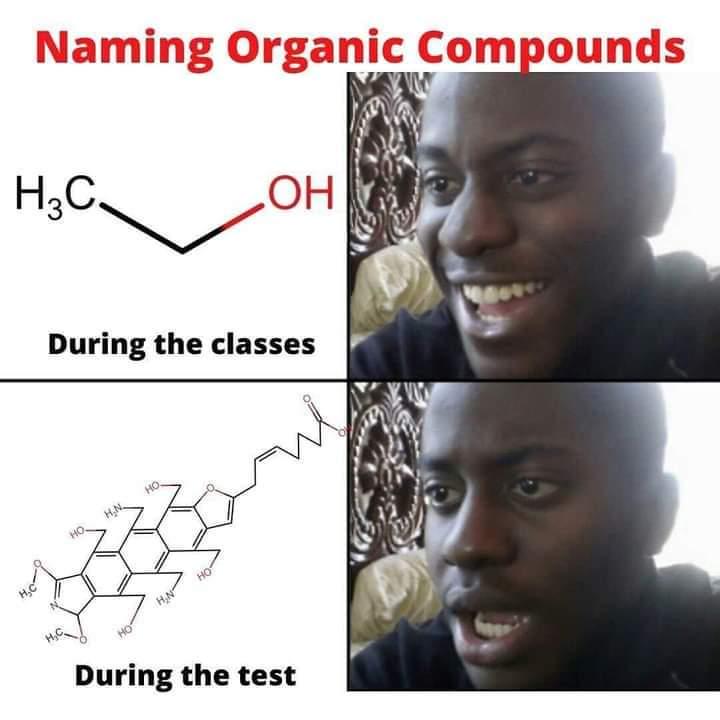
The Jekyll and Hyde personality of fluorine captured perfectly! In inorganic chemistry, fluorine is that psychotic werewolf ready to violently react with basically anything. It's the element that makes chemists back away slowly while maintaining eye contact. Meanwhile, in organic chemistry, fluorine transforms into this friendly golden retriever that just wants to hang out in your molecule, stabilizing things and barely reacting at all. Same element, completely different behavior depending on the chemical neighborhood. Chemistry's ultimate split personality disorder - fluorine will either tear your lab apart or sit quietly in the corner. No in-between!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology