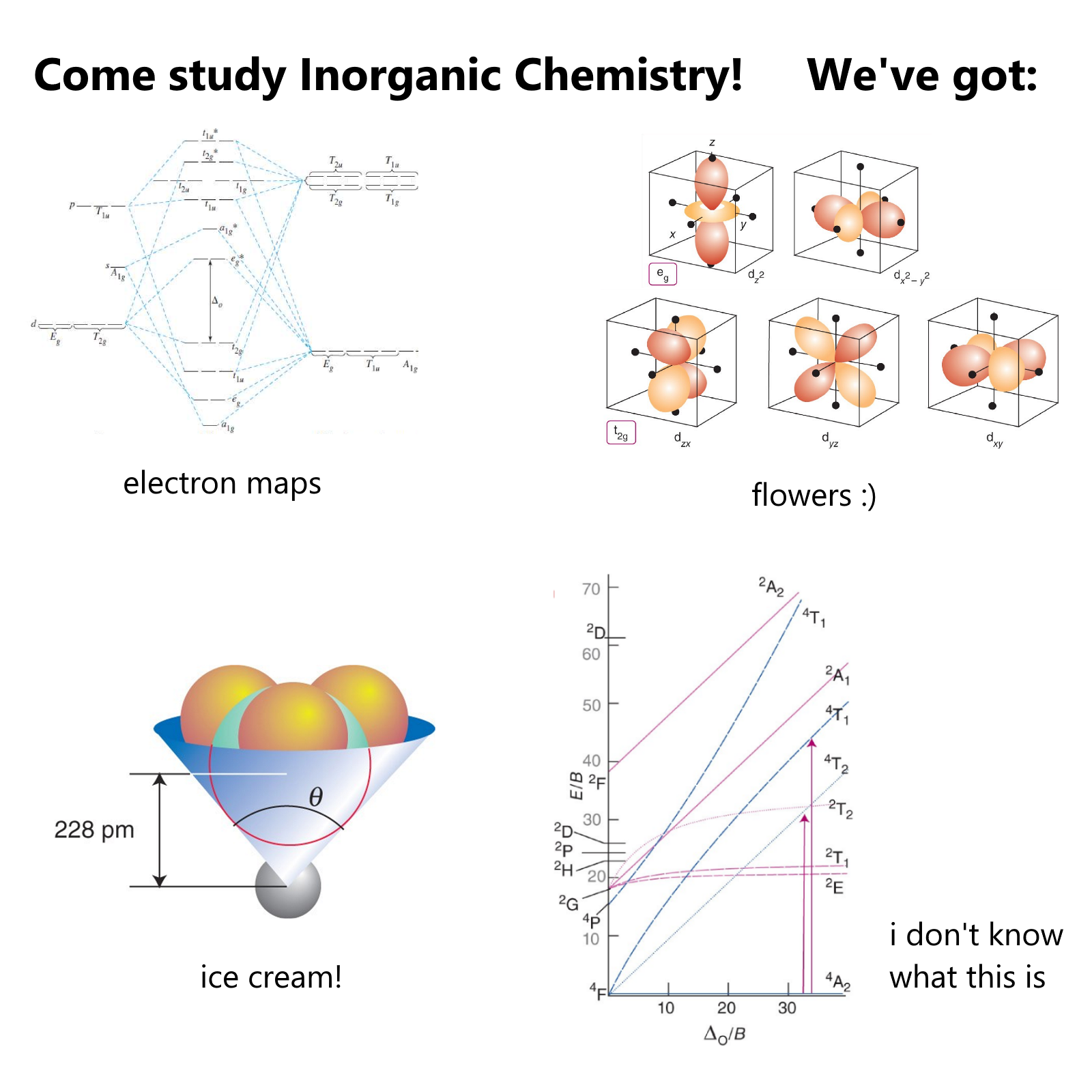
The ultimate chemistry student struggle! This meme perfectly captures the frustration of trying to understand molecular polarity with absolutely zero visual differences. Four identical cat pictures labeled with different polarity concepts (molecule, geometry, dipole moment, partial charge) brilliantly satirizes those textbooks that expect you to magically visualize electron distribution without proper illustrations. It's basically like saying "Here's the exact same information four times - now understand the difference!" Chemistry professors be like: "It's obvious, just FEEL the electronegativity!" 😂


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology