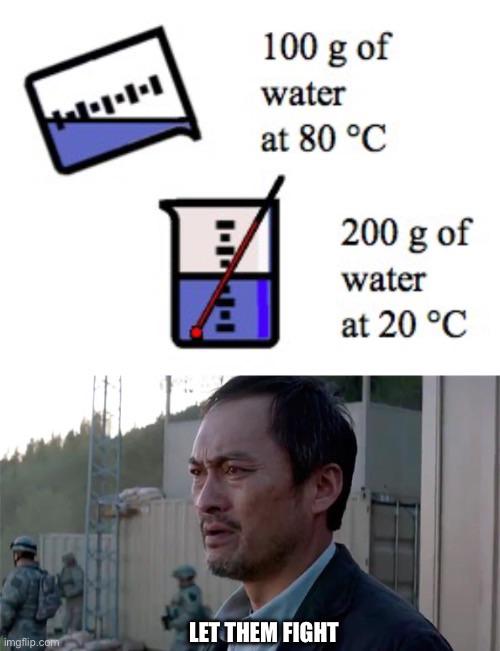
The eternal battle between hot and cold water is about to get real. What we've got here is a classic thermodynamics cage match - 100g of hot-headed water at 80°C being poured into 200g of chill, room-temperature water at 20°C. The guy's just standing back like every physics professor who's secretly enjoying the chaos of an experiment gone wild.
The final temperature will settle somewhere around 40°C because energy can't be created or destroyed, just transferred from the hot-shot molecules to the lazy cold ones. It's like watching that one overachiever in the group project carrying everyone else.
Every thermodynamics student has that moment when they realize nature always finds equilibrium whether you like it or not. No amount of rooting for the underdog will change the math!

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology