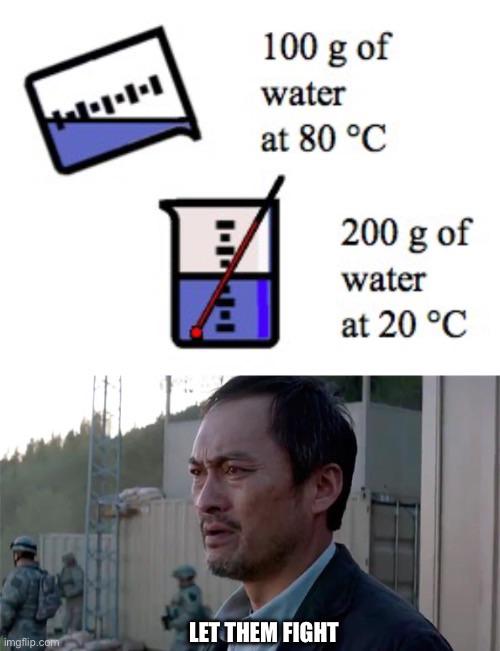
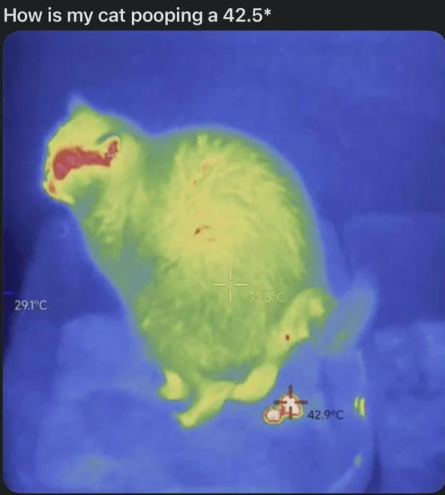
The eternal struggle of engineering students vs. dimensionless numbers! That Heat Transfer professor has introduced the Reynolds, Nusselt, Prandtl, Grashof, and now—BAM—here comes another one! These pesky ratios with no units are the bane of thermal analysis. Students frantically scribbling Pi groups while the professor casually drops another Biot number like it's nothing. The mental breakdown is imminent! Next person who says "just use the Buckingham Pi theorem" might find themselves in a strongly exothermic reaction with my patience!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology