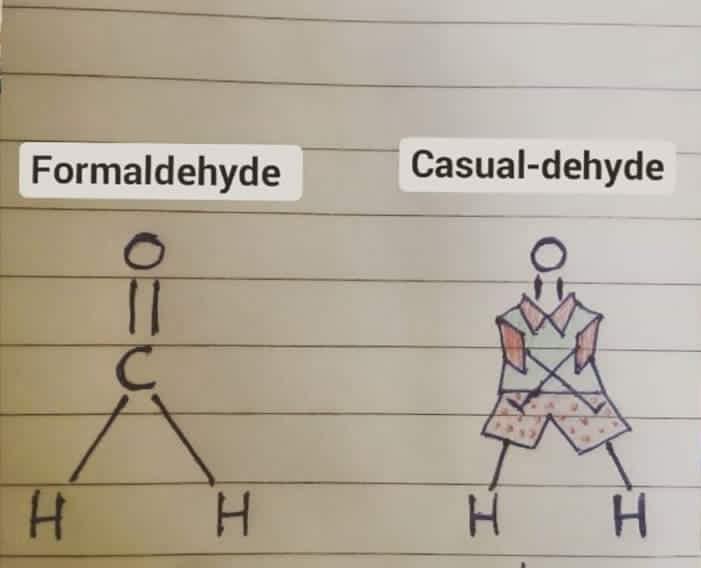
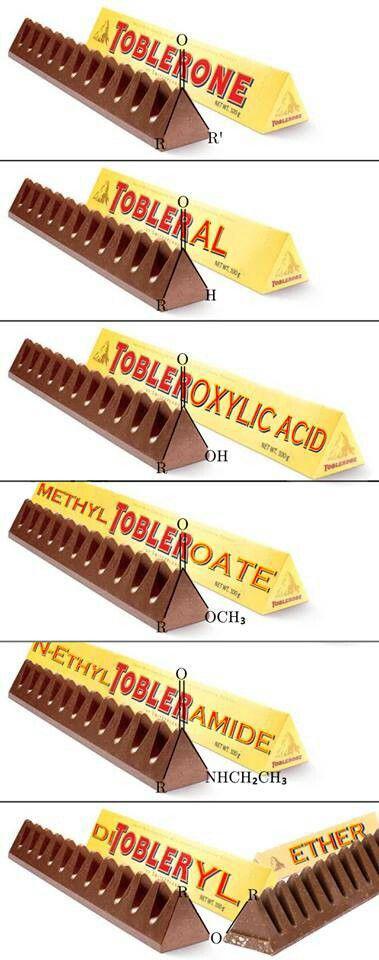
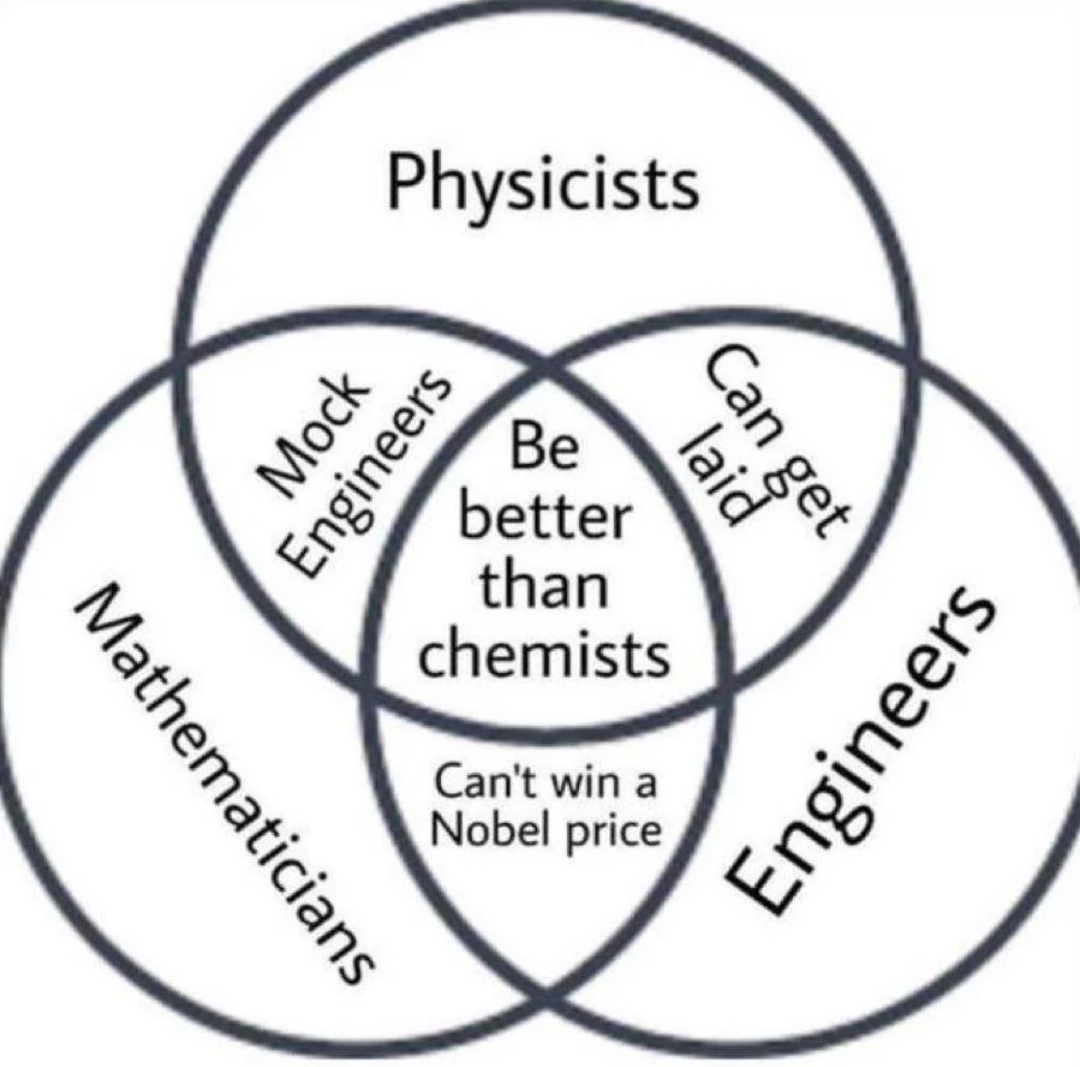
The eternal struggle of chemistry students everywhere! When you mix 50mL of ethanol with 50mL of water, you'd think you'd get 100mL of solution because... math. But nope! Thanks to molecular interactions between ethanol and water molecules, the total volume contracts to around 96mL.
This phenomenon is called "volume contraction" and happens because the smaller water molecules can nestle into spaces between the ethanol molecules. It's like trying to fit both basketball players and jockeys into an elevator - they pack more efficiently together than separately!
The confused bird's double "WHAT?" perfectly captures every first-year chem student's brain short-circuiting when they measure their final solution and think they've somehow spilled 4mL. Trust me, no one escapes the existential crisis of "where did my volume go?!"

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology