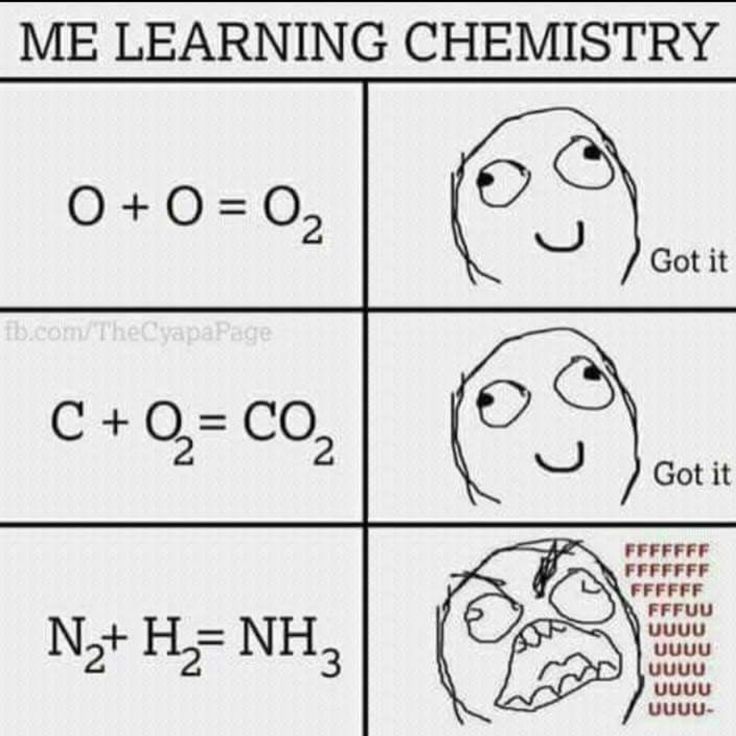
The beautiful journey of chemical education, where everything makes perfect sense until it suddenly doesn't. Simple diatomic oxygen formation? Easy. Carbon dioxide? Child's play. But then stoichiometry throws a curveball with nitrogen and hydrogen making ammonia, and suddenly you're questioning your life choices.
That third equation is where chemistry stops being addition and starts being a sadistic puzzle. N₂ + H₂ = NH₃? Where did that extra hydrogen come from? The balanced equation should be N₂ + 3H₂ = 2NH₃, which is precisely when most students transition from "I understand chemistry" to "I will become an English major."

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology