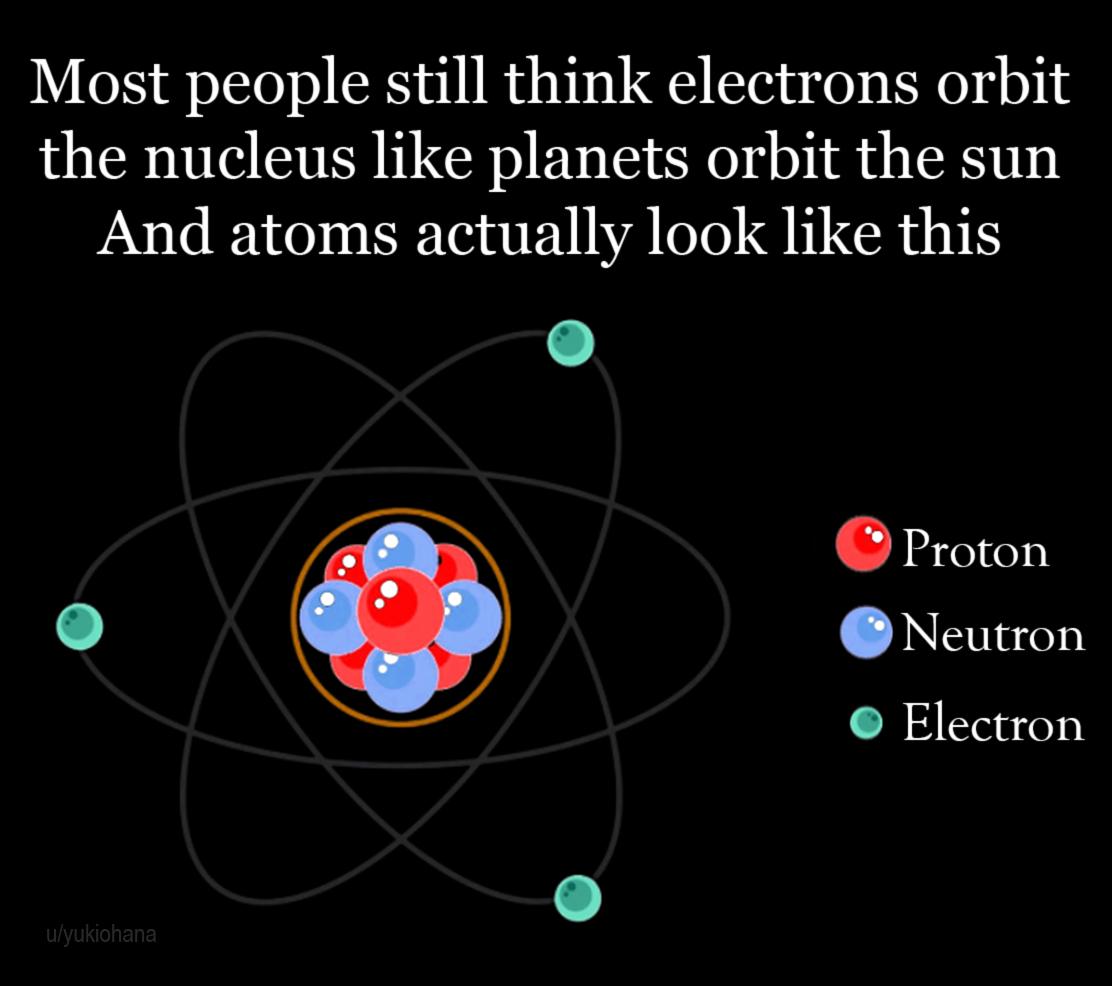
Congratulations! You're looking at the most persistent scientific misconception since we stopped believing the Earth was flat. That cute little planetary model of an atom? Pure fiction. Electrons don't circle the nucleus like obedient little planets—they exist as probability clouds in quantum states that would make Newton weep into his apple cider.
Thanks to pop culture and every science textbook illustration ever, we're stuck with this adorable but wildly inaccurate mental image. The reality? Electrons are more like moody teenagers—impossible to pin down exactly where they are and what they're doing at any given moment. Quantum mechanics is nature's way of saying "your intuition is cute, but wrong."

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology