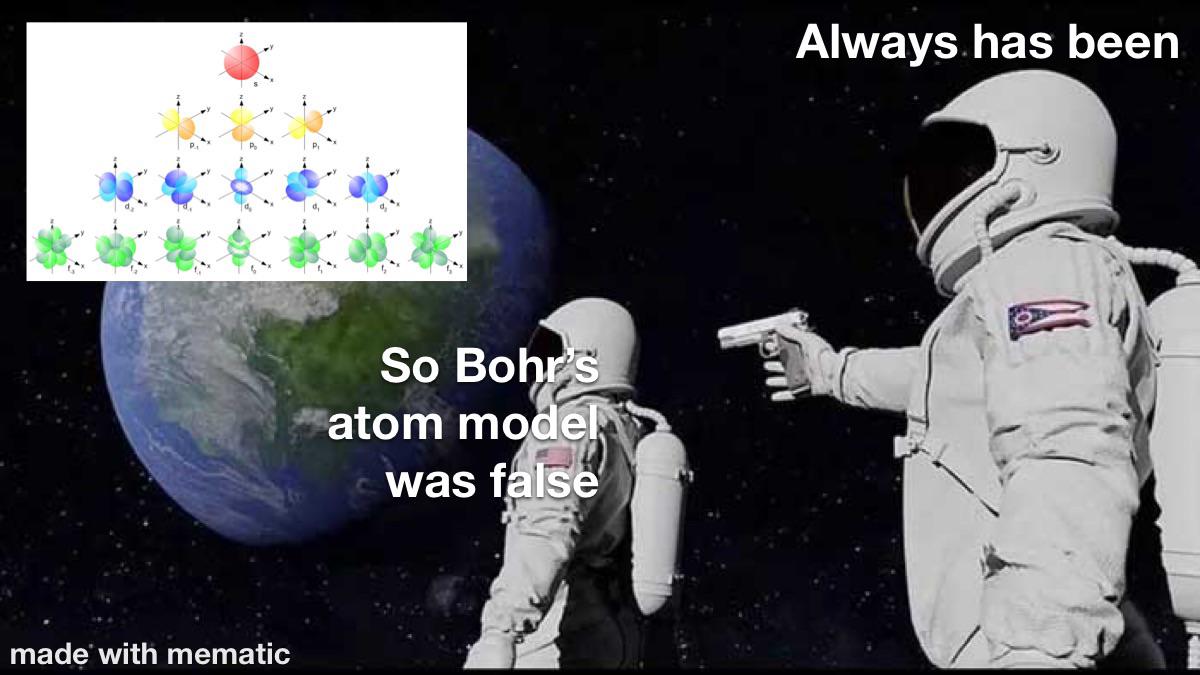
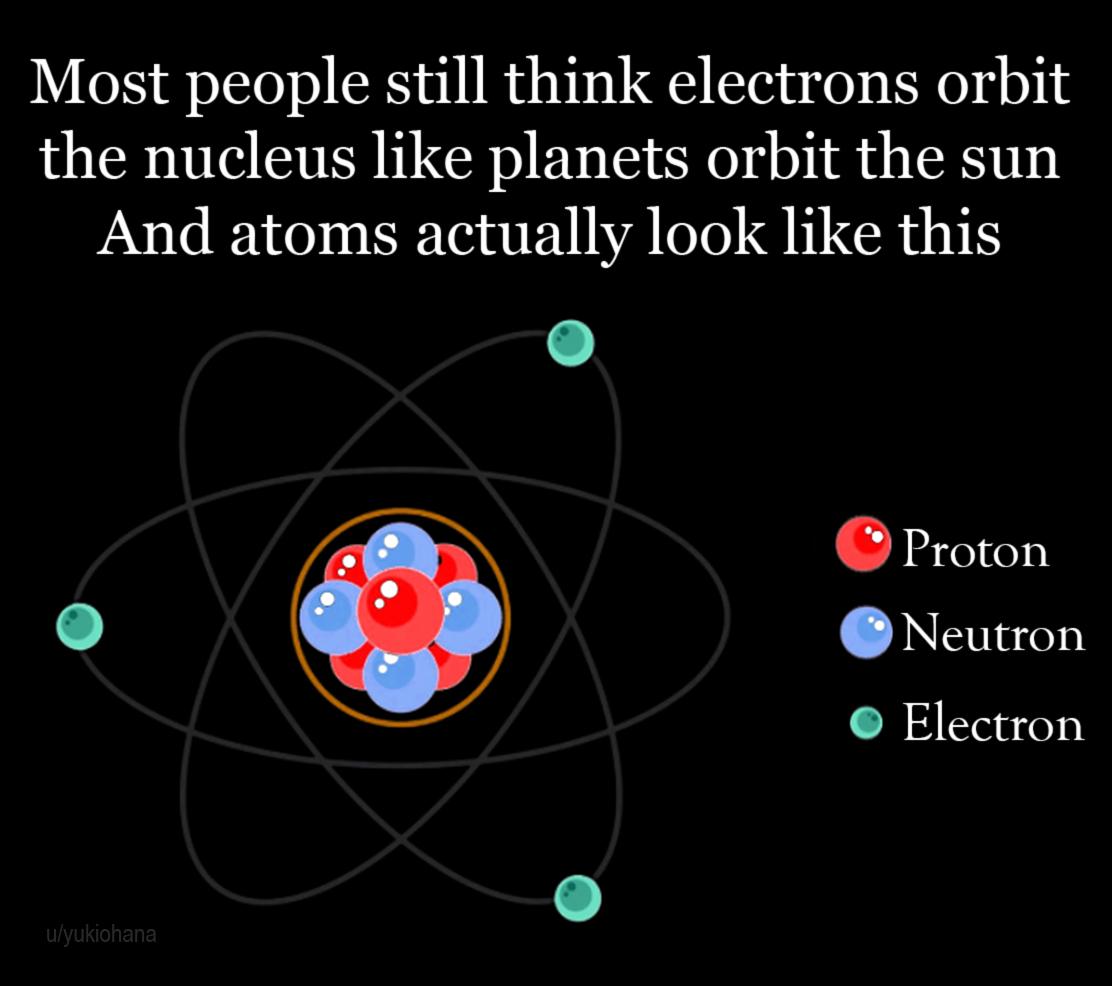
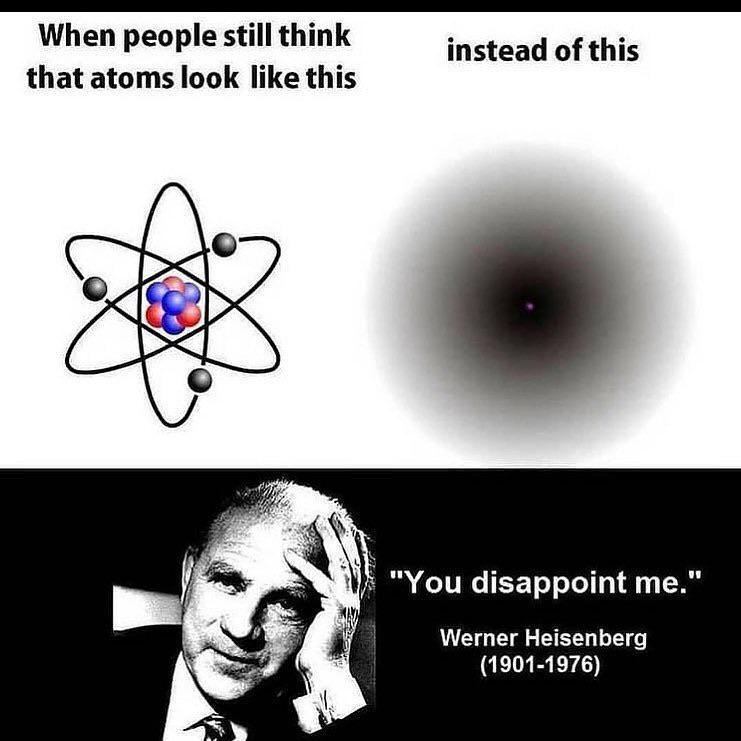
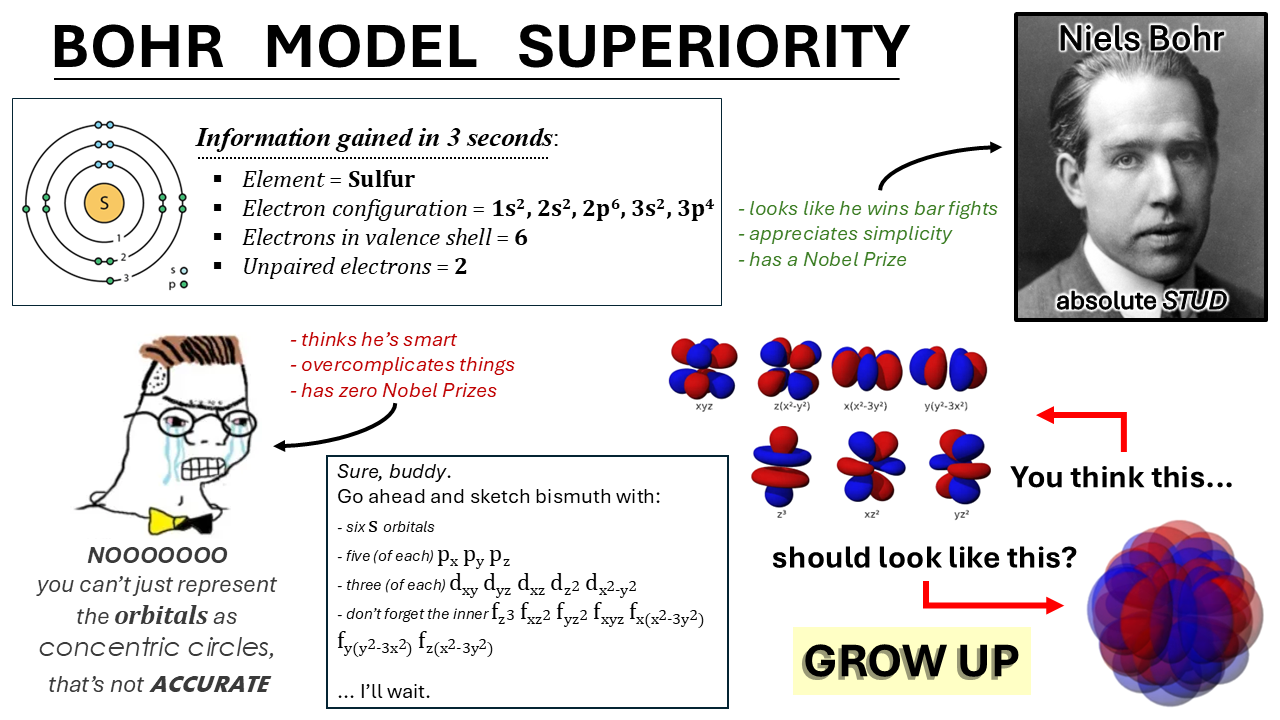
The eternal battle between simplicity and accuracy in atomic models. On one side, the Bohr model gives you sulfur's electron configuration in 3 seconds flat with neat little circles. On the other, quantum mechanics enthusiasts are having an existential crisis over orbital shapes, hybridization, and mathematical functions that look like someone sneezed on a keyboard. Sure, electron probability clouds are more "accurate," but can they tell you how many valence electrons you have before your coffee gets cold? No. This is why intro chem professors still draw those circles - they've seen the quantum abyss and chosen sanity instead.


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology