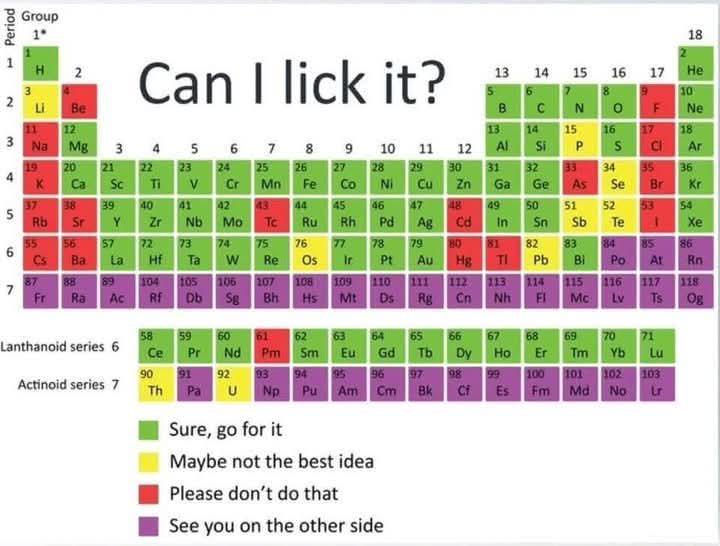
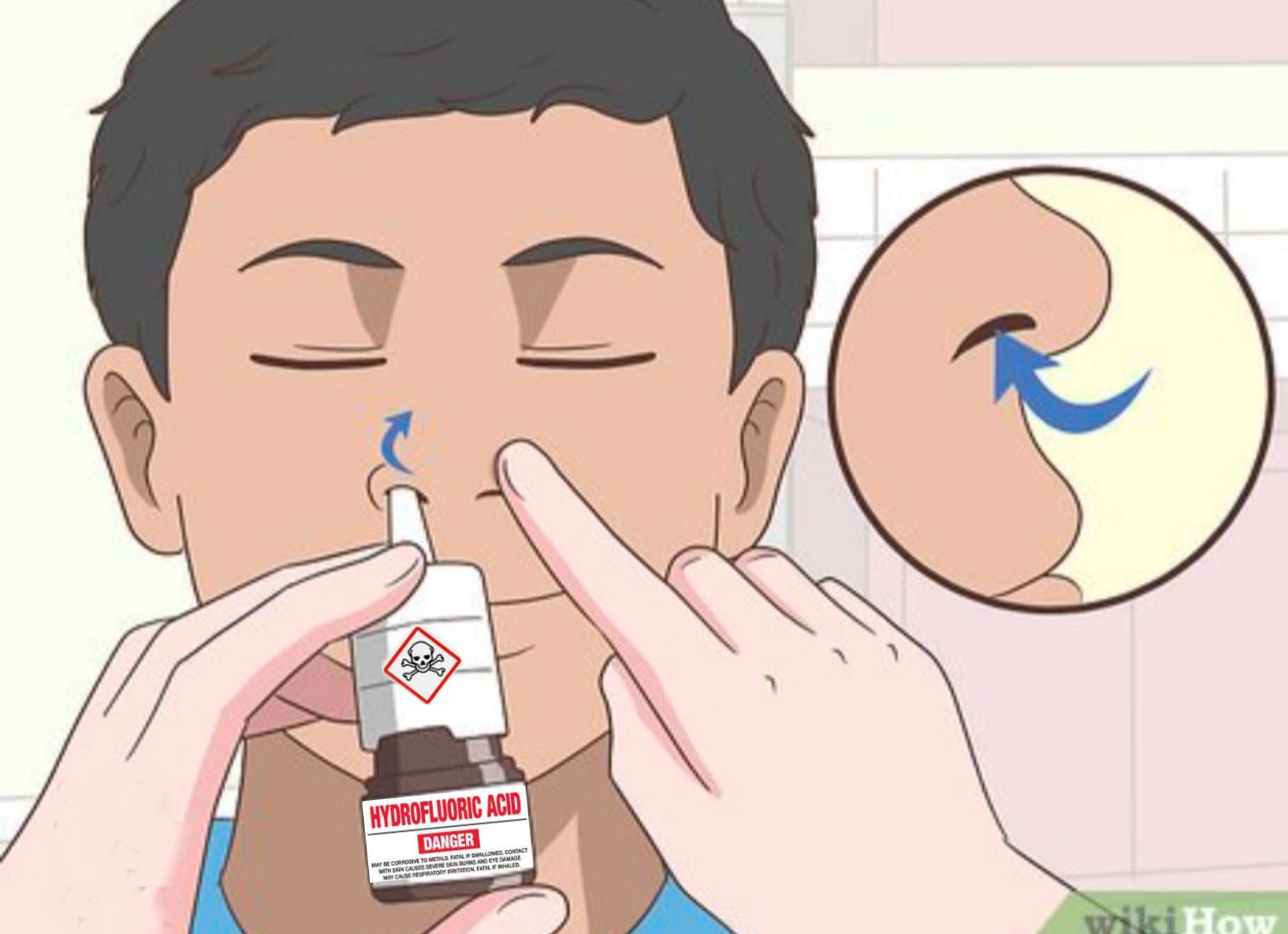
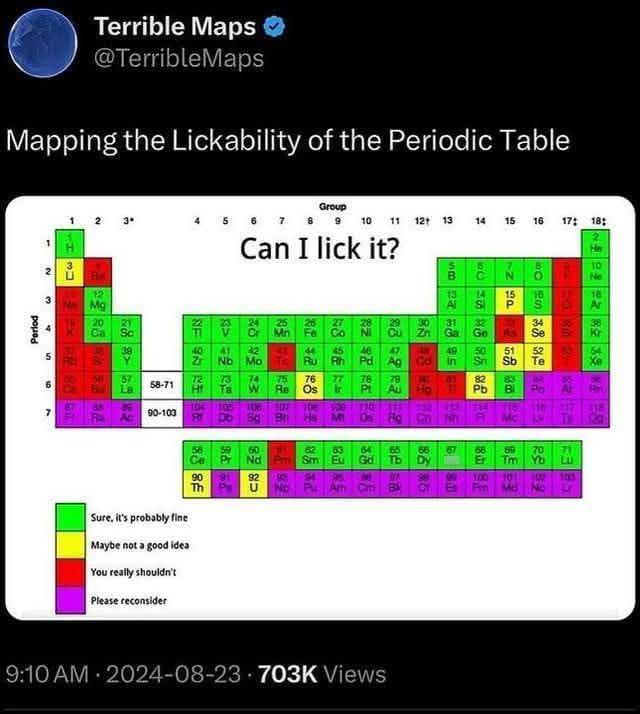
Chemistry enthusiasts gone wild! This meme showcases chlorine trifluoride (ClF3), possibly the most terrifying chemical compound ever created. Even Nazi Germany—who weaponized horrific chemicals—decided this one was TOO dangerous to use in warfare! ClF3 is basically chemistry's final boss. It burns at 2,400°C, converts to hydrofluoric acid (which dissolves your bones while you're still alive), and sets fire to things that shouldn't even be flammable—like concrete, asbestos, and even ash from previous fires! The contrast between the horrified WWII soldiers and our modern mad scientist is pure gold. When your chemical is too extreme for people who invented nerve gas, maybe reconsider your weekend hobby! 😂


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology