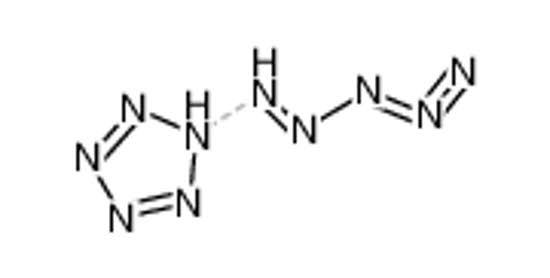
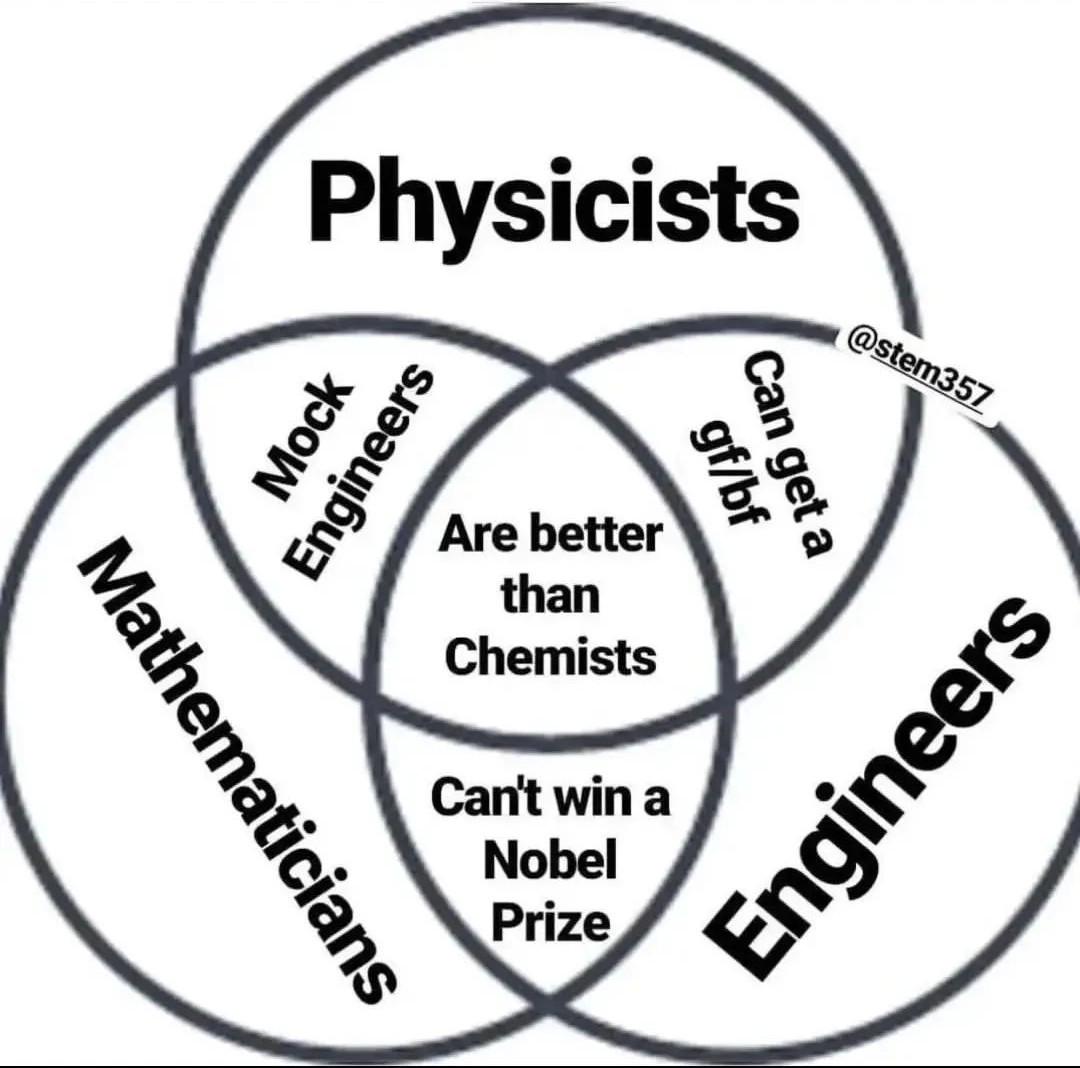
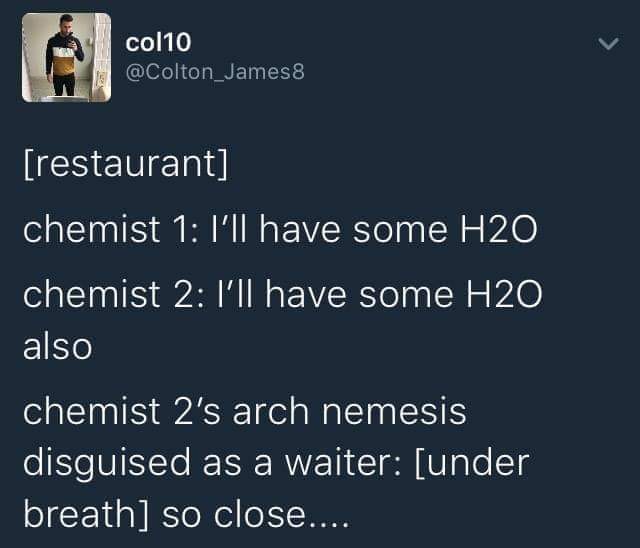
How many scientists does it take to change a light bulb? None, they're still writing the grant proposal.
Ad nuphy Air96 V2 Keyboard
Clickety-clack your way to keyboard nirvana

Your purchase contributes to our 'Why Is This Simple Chemical Reaction Not Working' therapy fund. 💔

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology