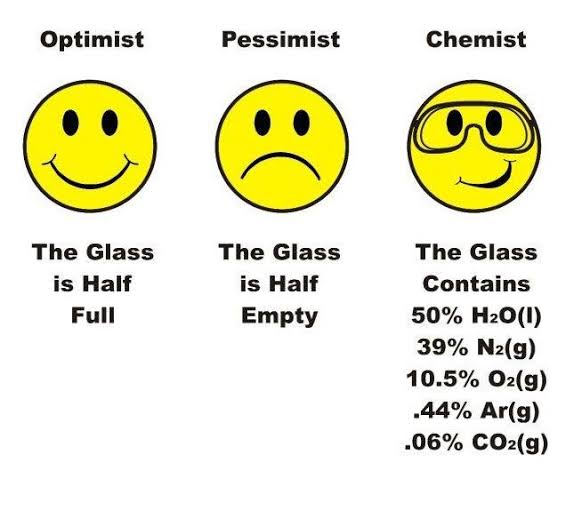
Water molecules playing the ultimate chemical mediator! The meme perfectly captures what happens in acid-base neutralization reactions. First panel: Water proudly declares "all acids and bases removed" like some overconfident bouncer at a chemical nightclub. Second panel: Other water molecules are horrified at this blatant lie. Final panel: The truth emerges - water didn't eliminate anything, it just created hydronium (H₃O⁺) and hydroxide (OH⁻) ions, bringing the reaction to equilibrium.
This is basically every chemistry student's moment of revelation when they realize water doesn't actually "neutralize" acids and bases - it just transforms them into a balanced state where they can coexist without causing chemical drama. Chemistry: where nothing truly disappears, it just changes its relationship status to "it's complicated."

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology