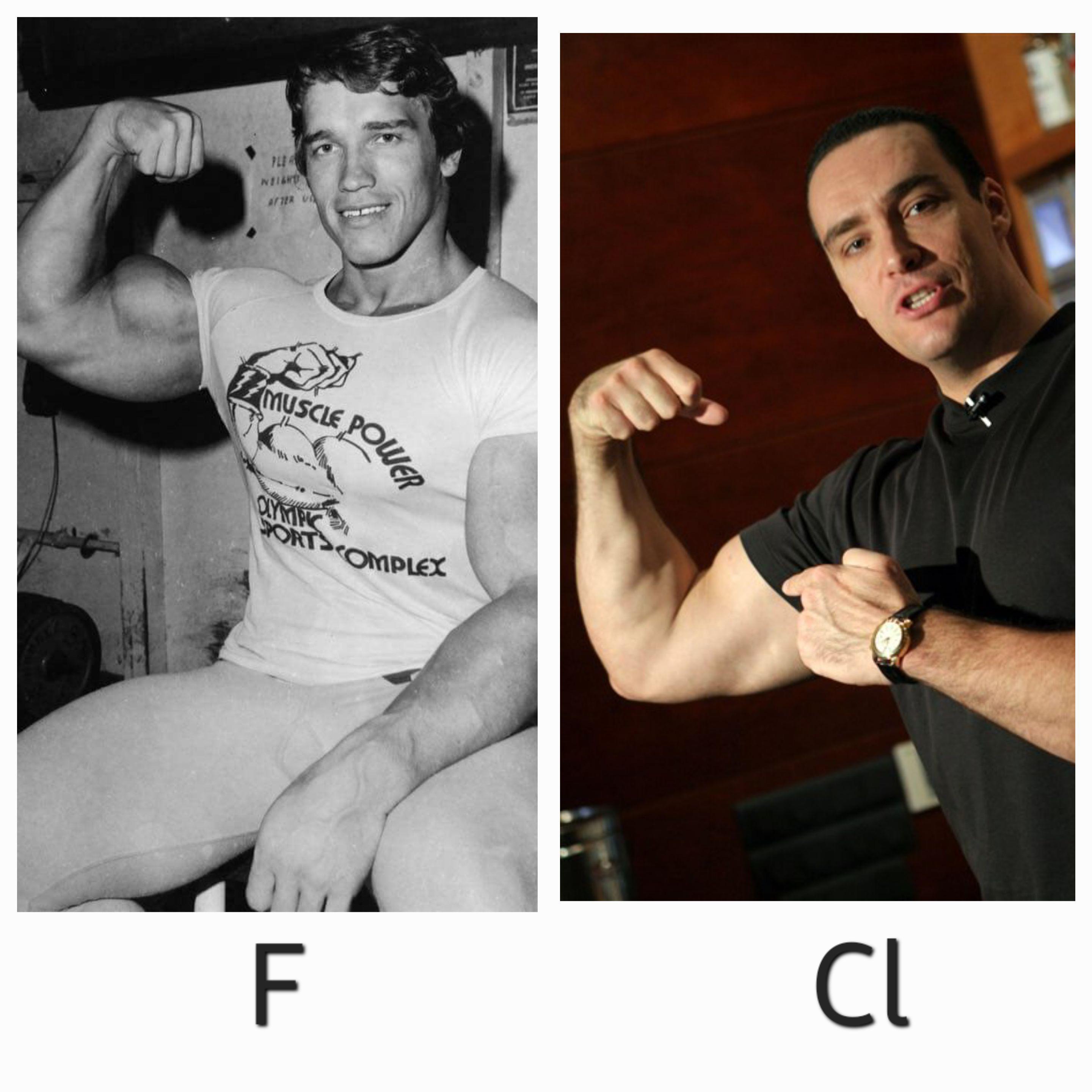
Ever notice how the periodic table is basically just a gym for electrons? This brilliant chemistry joke compares muscular individuals to Fluorine (F) and Chlorine (Cl) - two elements that are just one electron away from having a full outer shell and achieving stability!
Fluorine is super reactive and will literally STEAL an electron from almost anything to get buff (stable). Meanwhile, Chlorine is slightly less aggressive but still desperately wants that extra electron to complete its valence shell. Both elements are basically the gym bros of the halogen family - flexing their electron-attracting powers!
And just like how these two muscular figures might look similar at first glance but have different "strengths," F and Cl have different electronegativities! Fluorine is the most electronegative element in the entire periodic table - it's basically the ultimate electron thief! 💪⚗️

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology