Ad SAMSUNG 32" 4K Curved
Pixels so sharp they'll cut your eyestrain

Your purchase helps us pay for the extra lab assistants we need during those unexpected experimental breakthroughs. 📈

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
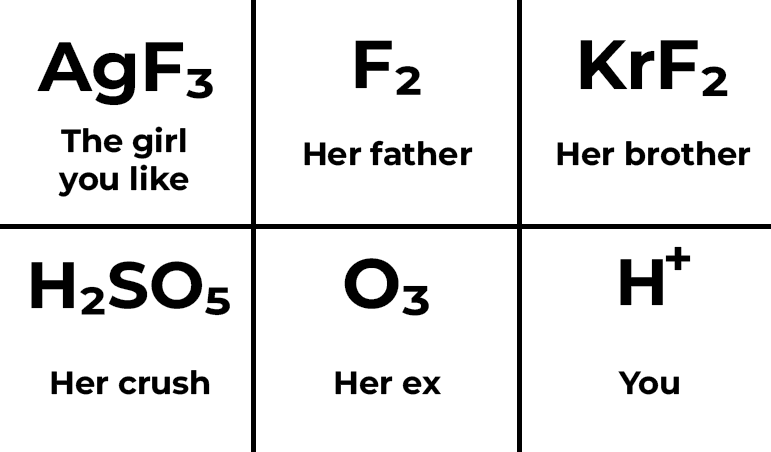
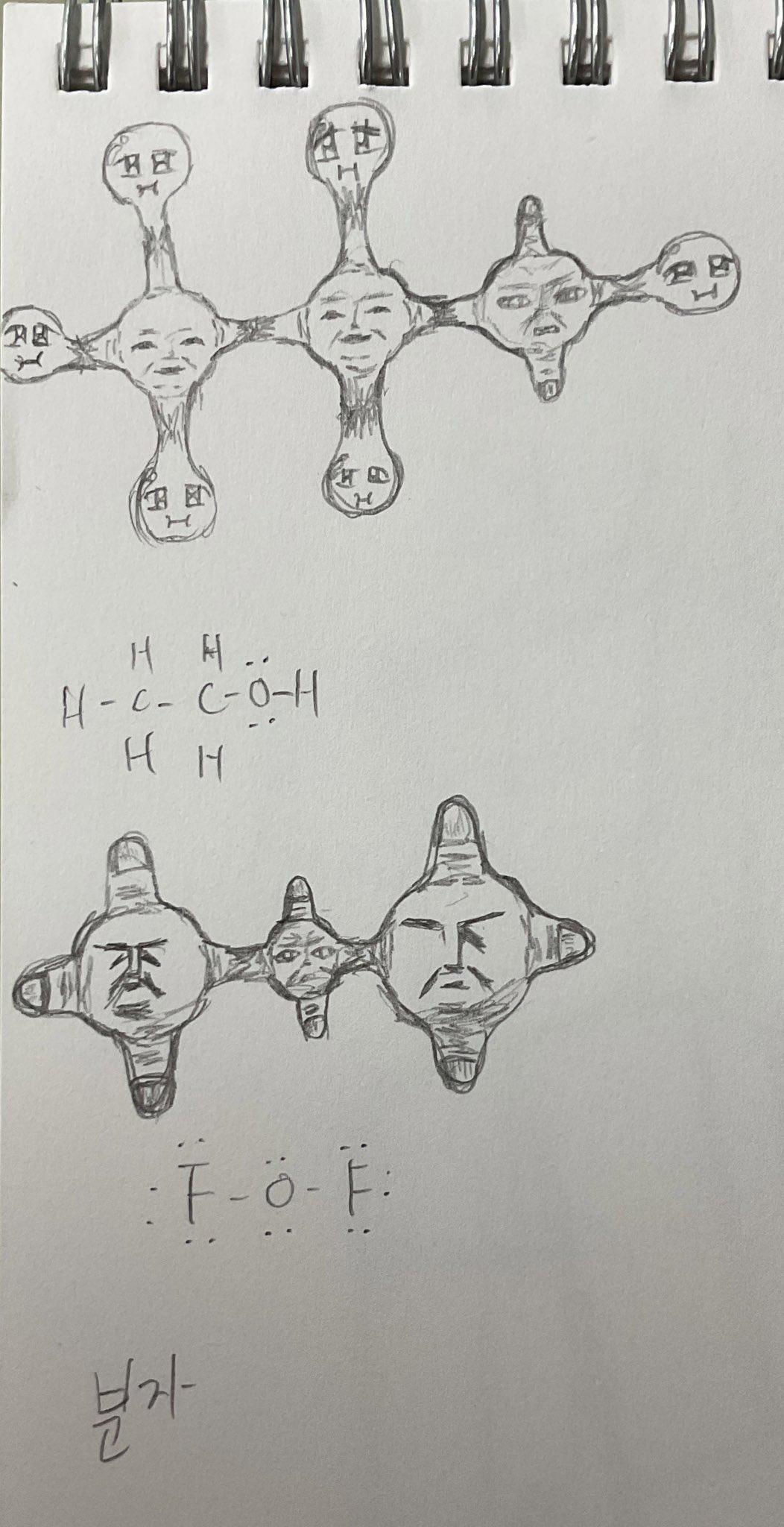
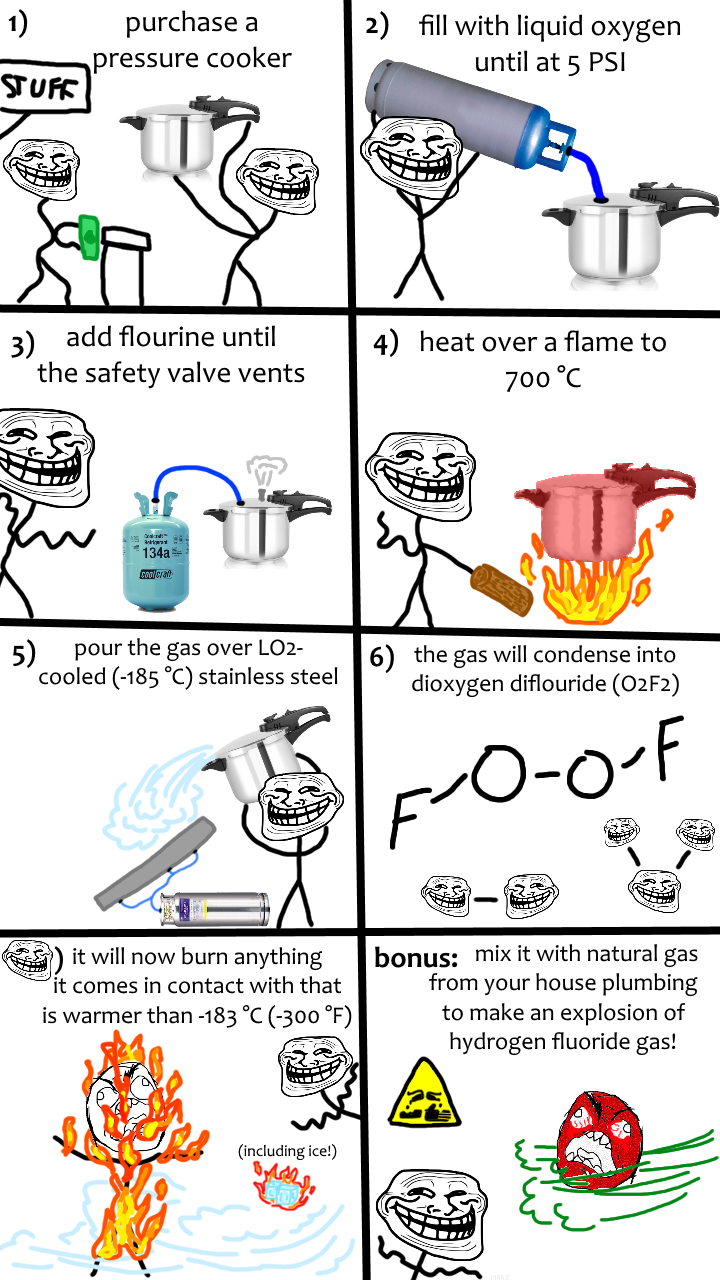
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology