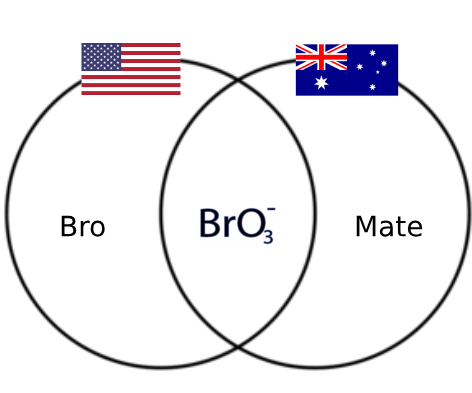
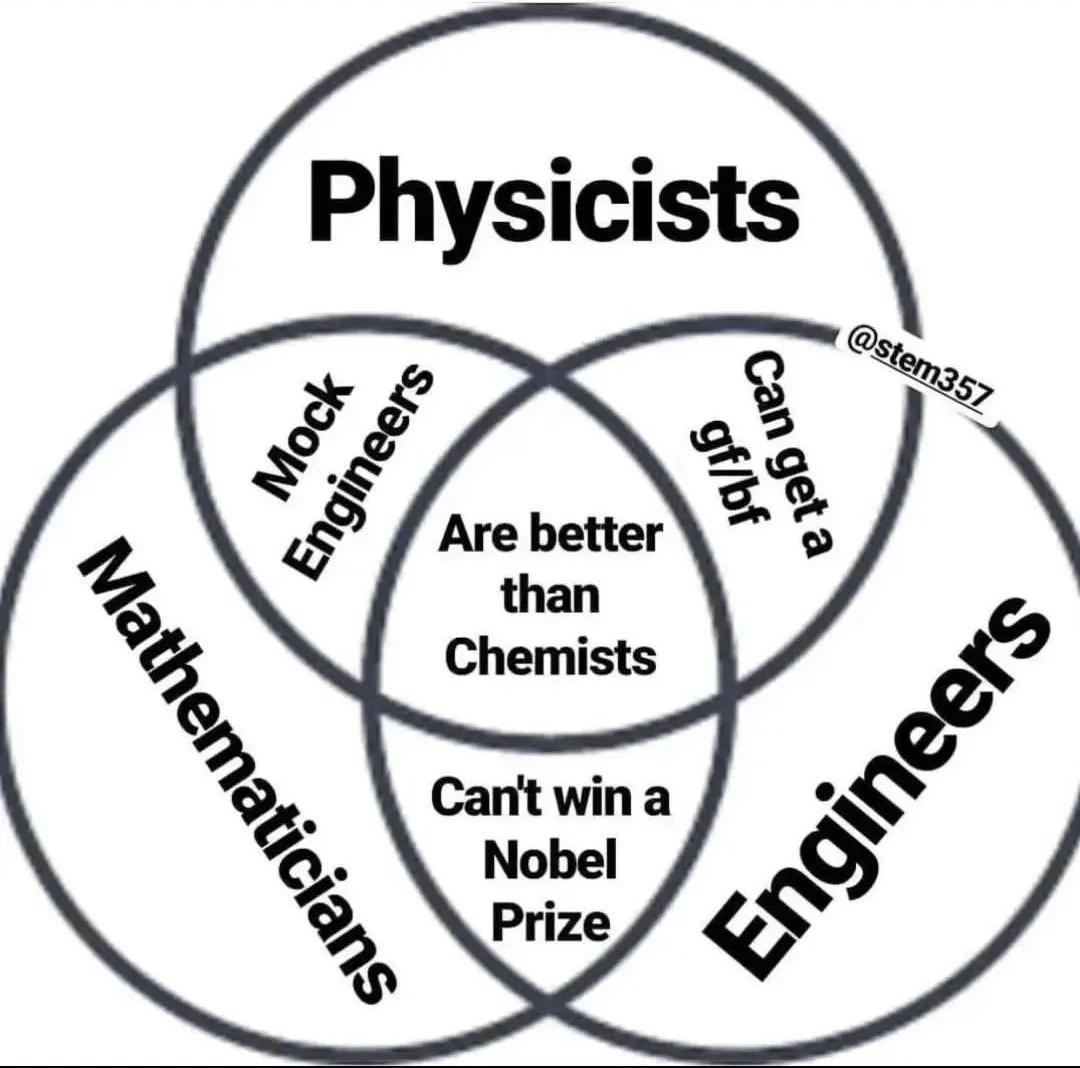
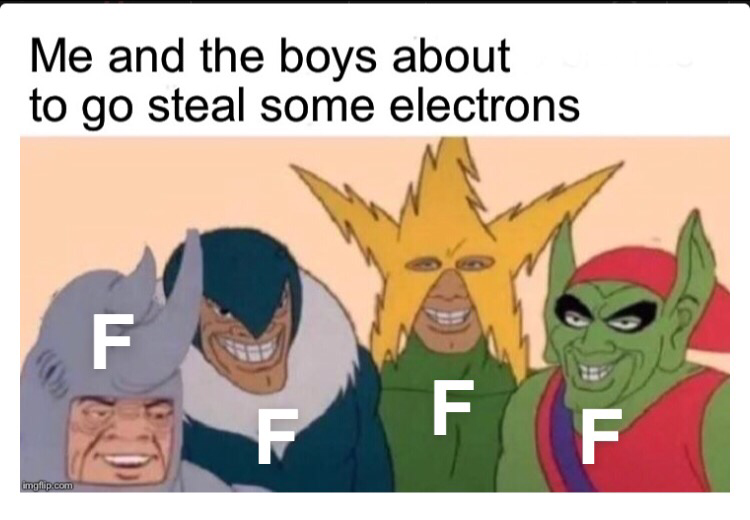
Oh snap! The periodic table just went full Star Wars on us! This chemistry crossover is giving us transition metal drama worthy of the Jedi Council. The d-block elements (Co, Mn, Cu, Fe, V, Ni, Cr) are basically the cool kids table of the periodic table, sitting there with their partially filled d-orbitals, judging poor Scandium and Zinc for being... basic. 😂
See, Sc and Zn are technically in the d-block but they're the awkward oddballs - Scandium has just ONE electron in its d-orbital, while Zinc has a FULL SET of d-electrons. Neither exhibits the classic "transition metal behavior" that makes the others so special. They're basically the chemistry equivalent of showing up to the Sith party wearing a Hello Kitty backpack.
Chemistry gatekeeping at its finest! The periodic table has cliques too, and these two elements just got DENIED.

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology