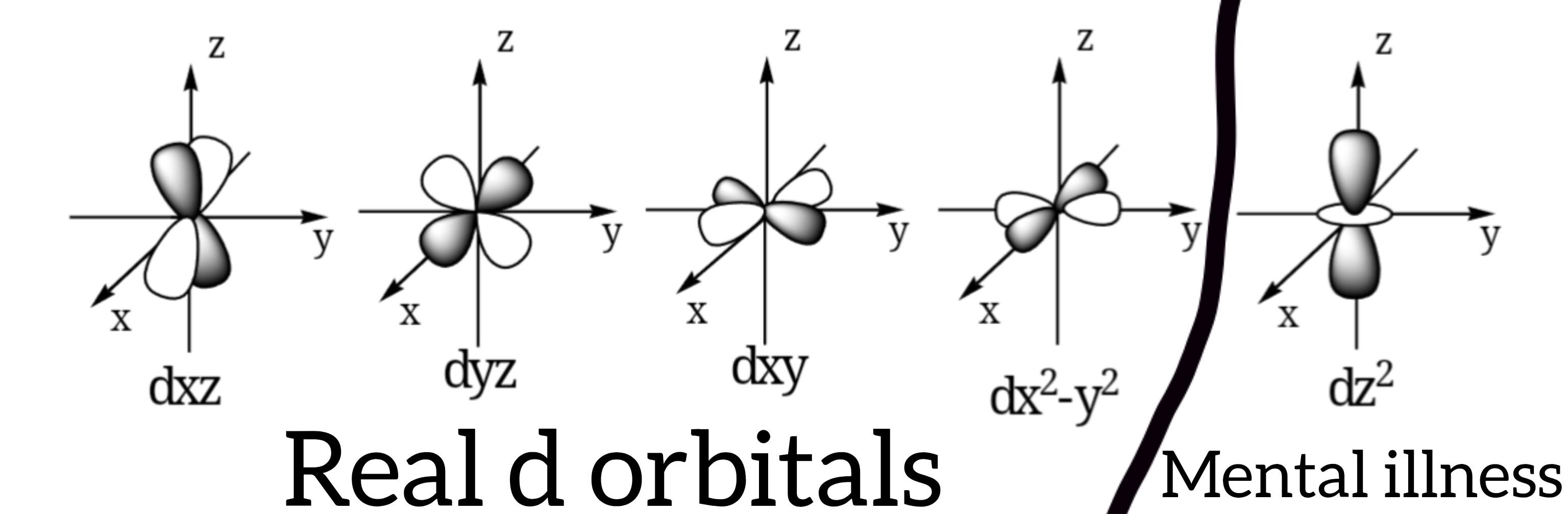
That moment when everyone else is making small talk but you're mentally calculating whether hydrogen should be considered a halide! The social skills of chemists are inversely proportional to their knowledge of periodic table debates.
While others discuss weather and sports, we're busy contemplating if hydrogen's electron-accepting properties qualify it for the halogen family. It's not our fault that pondering electronegativity is more interesting than whatever reality show everyone's watching!
Next time you're at a party, try dropping "hydrogen forms H- ions similar to F-, Cl-, Br- and I-" into conversation and watch everyone slowly back away. Worth it.

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology