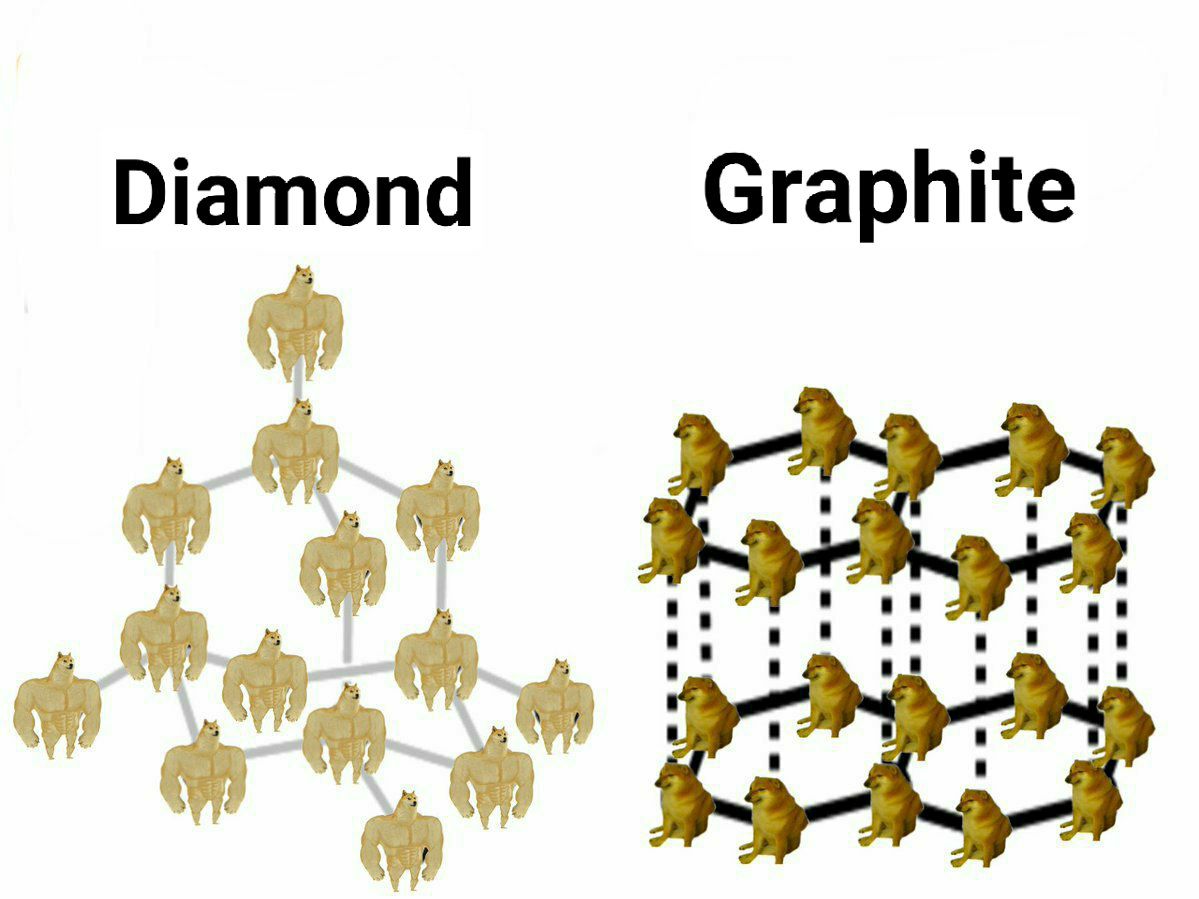
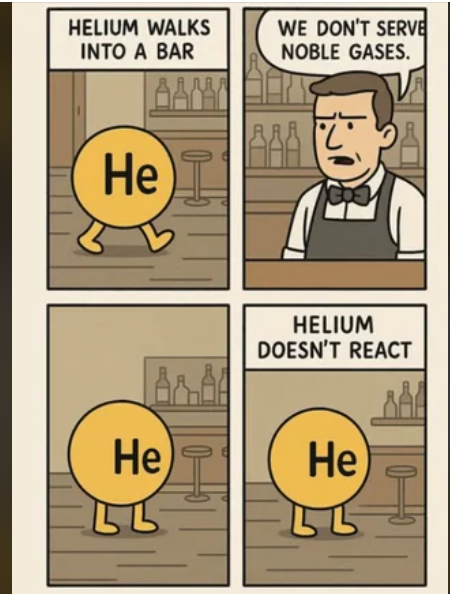
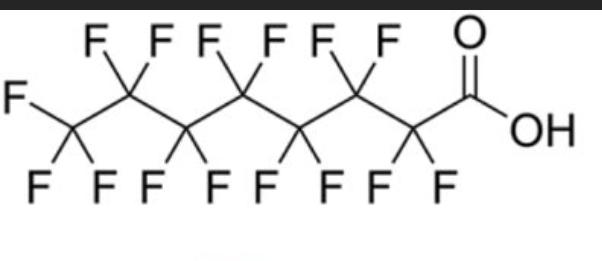
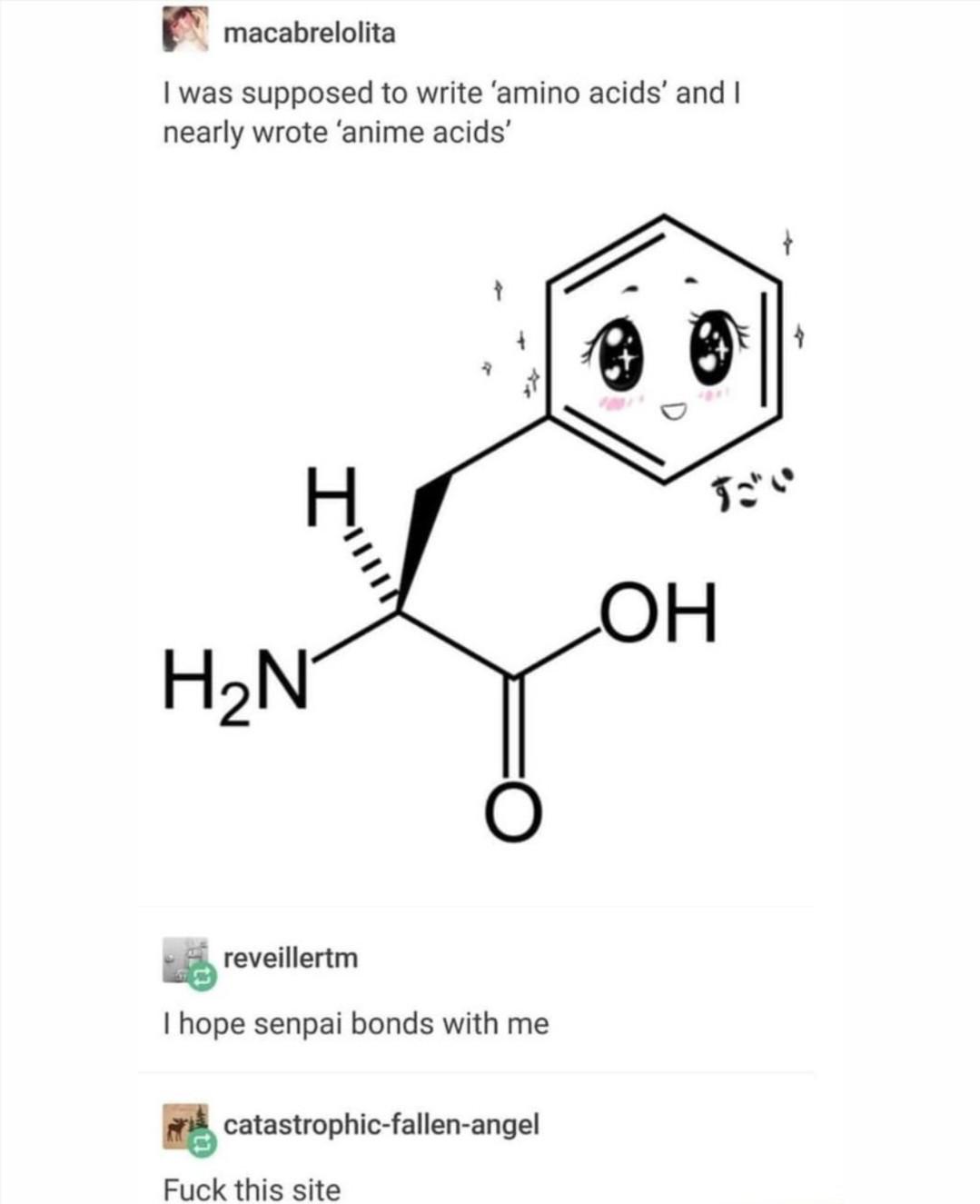
The perfect visual representation of carbon allotropes doesn't exi— 💎✏️
This meme brilliantly shows why diamond is the hardest natural material while graphite is what we write with! In diamond, each carbon atom forms strong bonds in a rigid 3D tetrahedral structure (represented by buff Doge), making it incredibly strong. Meanwhile in graphite, carbon atoms form sheets (regular Doges) that easily slide past each other – which is exactly why your pencil works!
Same element, completely different properties. Chemistry is basically carbon's personality disorder!

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology