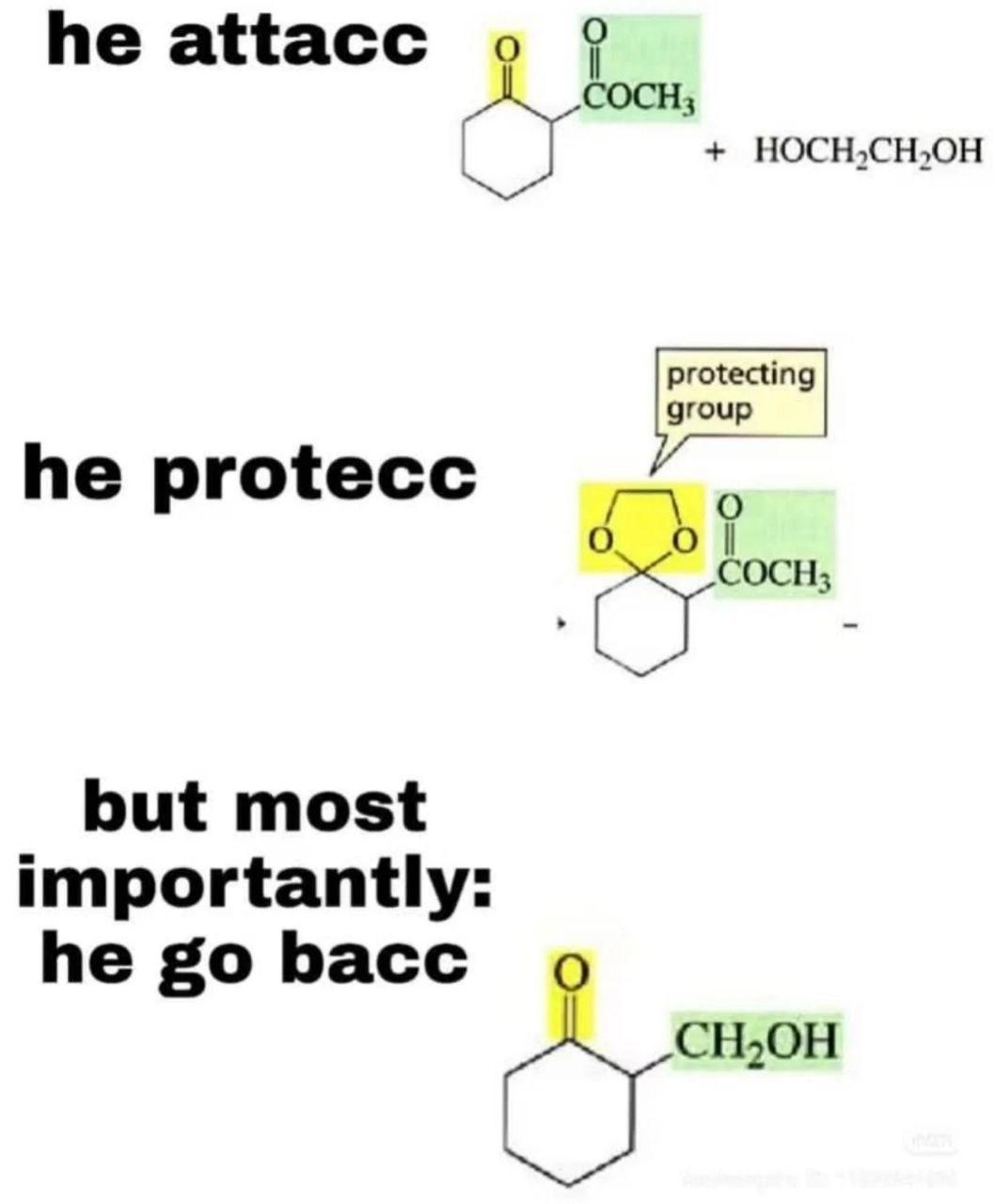
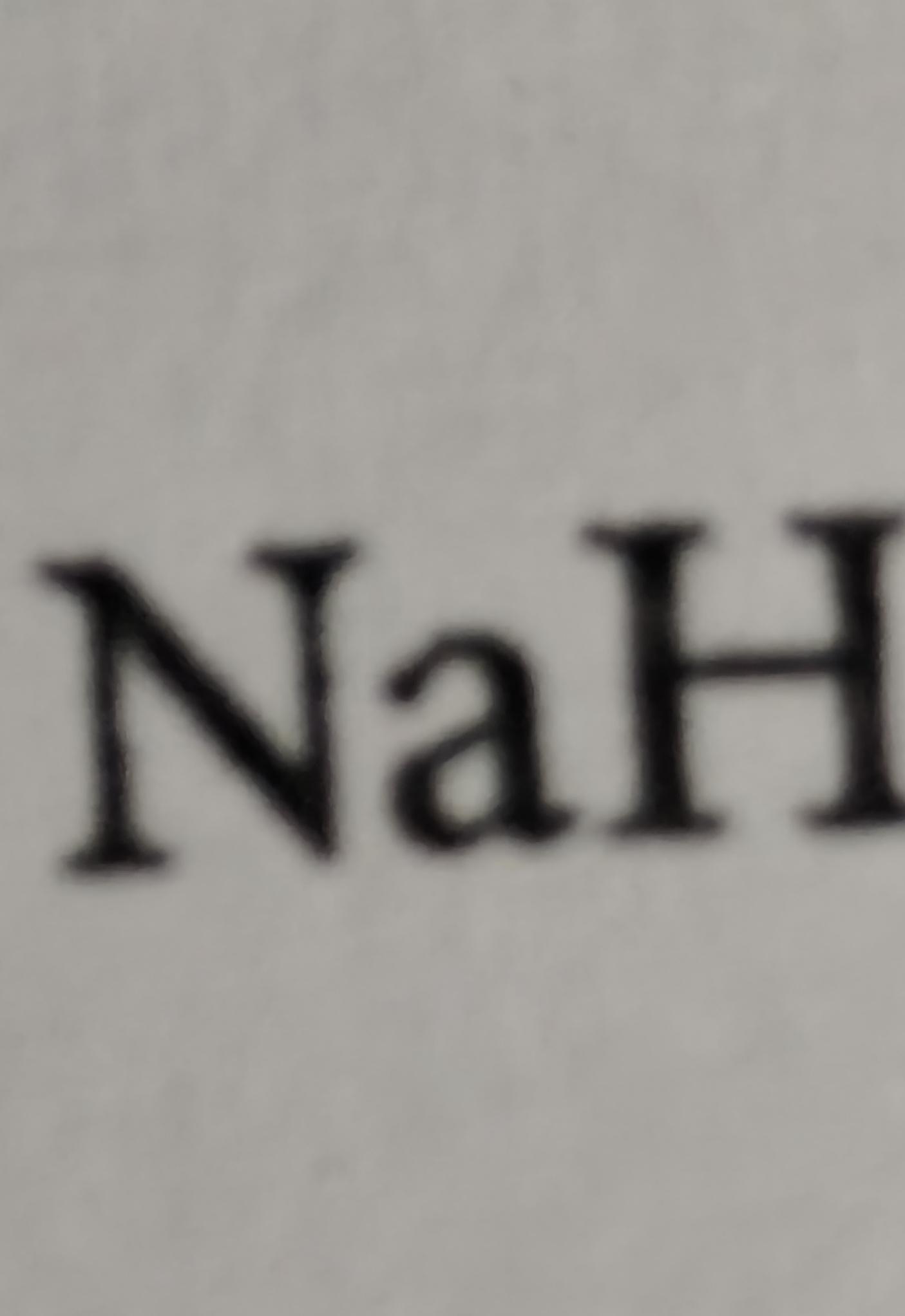The chemistry lab's most dramatic moment! The top panel shows various carbonyl compounds (aldehyde, ketone, carboxylic acid, etc.) hiding in a hallway like they're in some high-stakes action movie. Meanwhile, lithium aluminum hydride (LiAlH 4 ) bursts in like Darth Vader with a lightsaber, ready to donate those electrons and transform everyone. It's basically a chemical version of "I've come to reduce your double bonds and I'm all out of bubblegum." Those poor carbonyl groups never stood a chance against this reduction superstar - they're about to lose their oxygen and gain hydrogen faster than you can say "nucleophilic attack."


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology