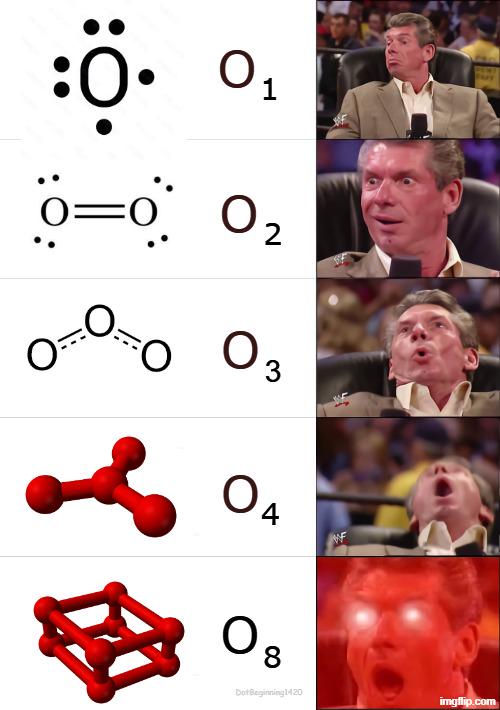
Oxygen is having an identity crisis! The meme shows oxygen's increasingly elaborate forms - from single atom O₁, to O₂ (what we breathe), to ozone O₃, to the less common O₄, until it goes FULL CARBON with that cubic O₈ structure! 😱 That last one is blowing minds because oxygen is basically copying carbon's cubic structure (like diamond). It's the chemical equivalent of your friend stealing your whole personality and pretending it was their idea all along! Fun fact: While O₂ keeps us alive and O₃ protects us from UV rays, that O₈ structure is super unstable and would probably explode if you looked at it wrong. Oxygen's midlife crisis is literally explosive!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology