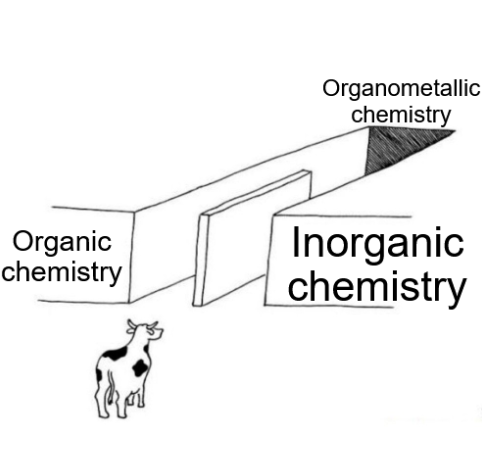
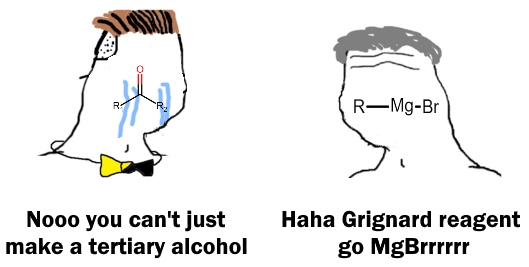
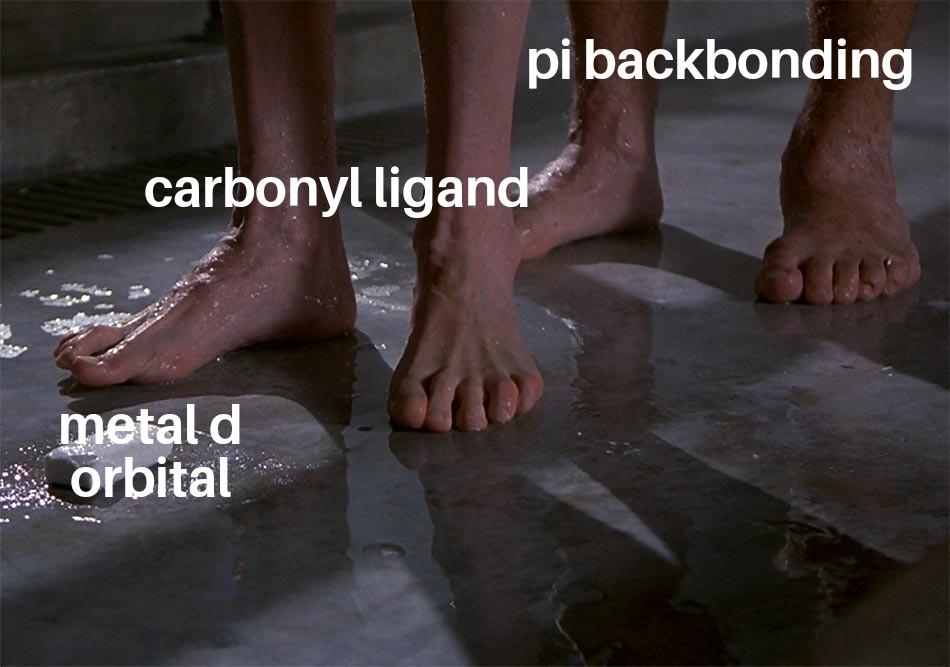
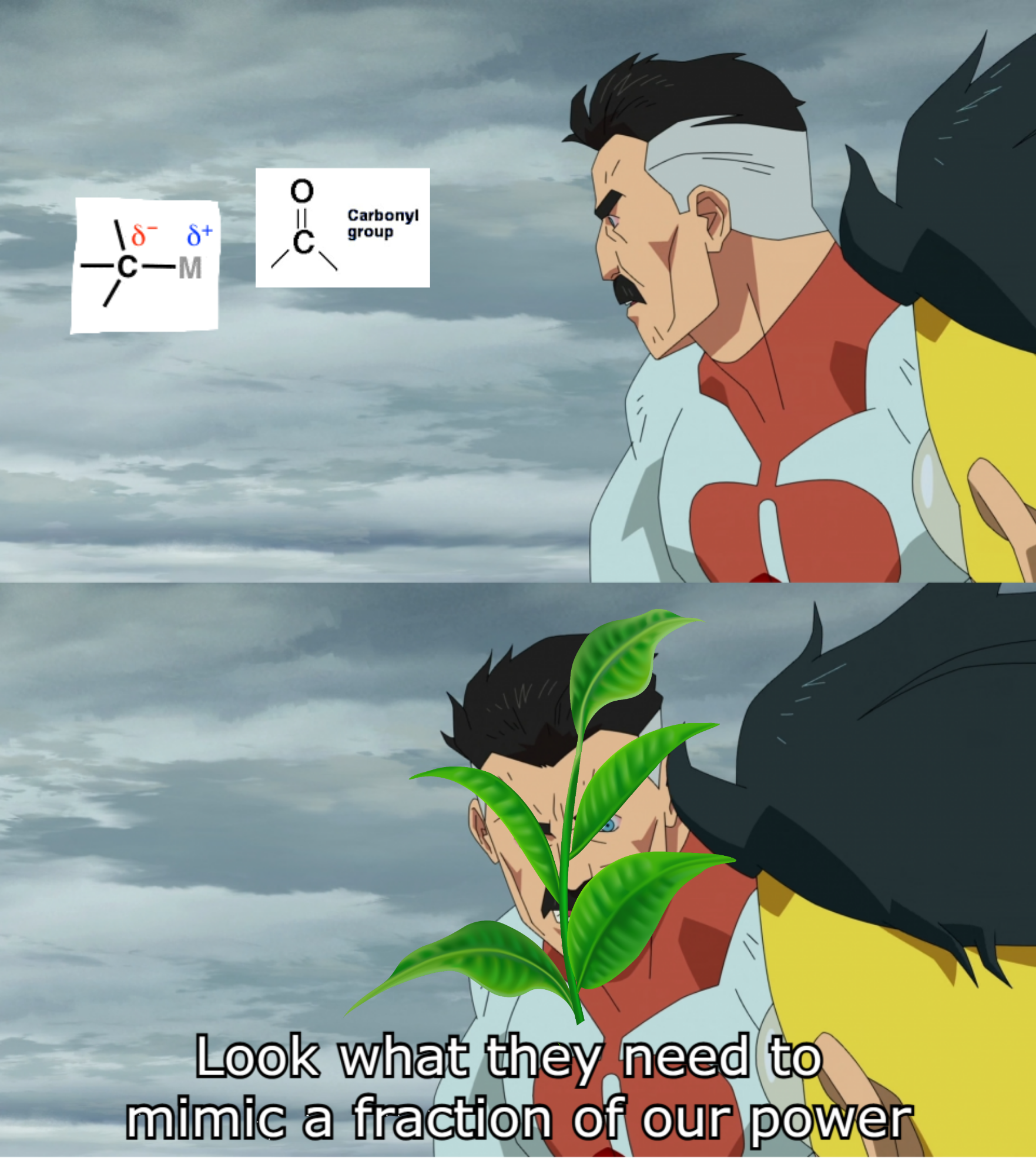
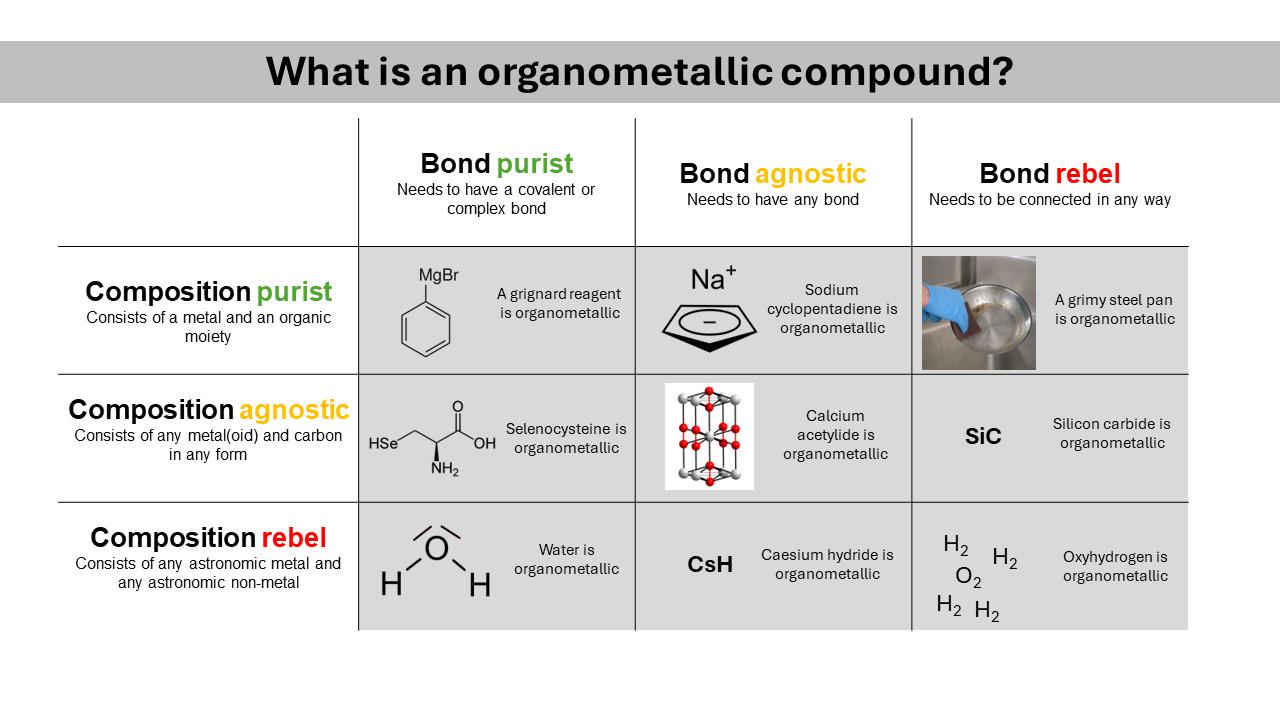
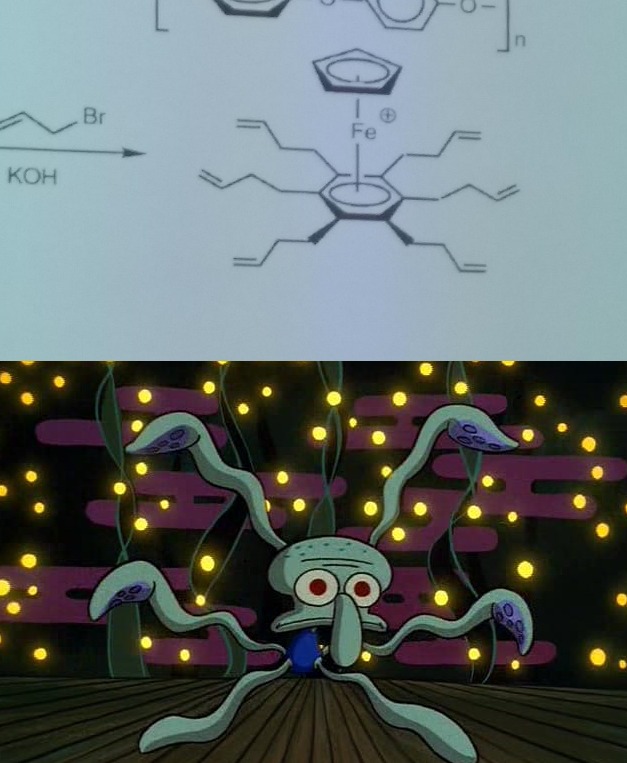
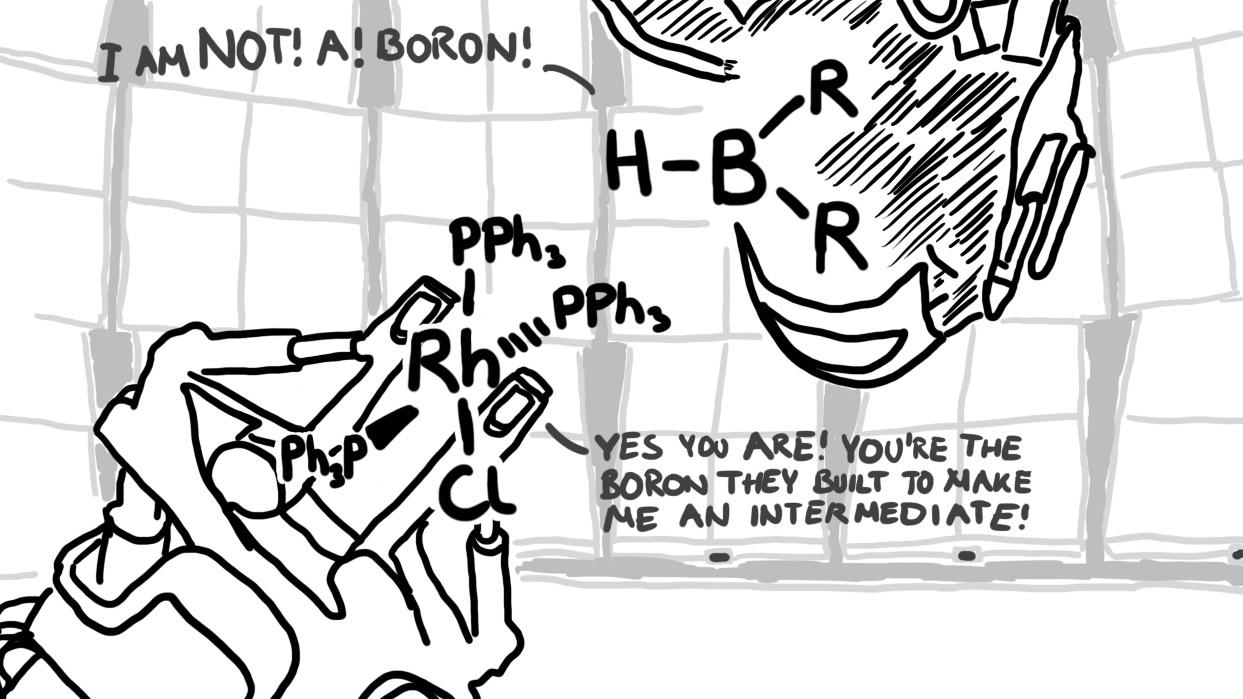
There are 10 types of people in this world. Those who understand binary and those who should study science.
Ad Breville Barista Express
Turn your kitchen into a hipster café

Buy = You get cool stuff + we can finally afford that electron microscope for ultimate magnification. 🔍

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology