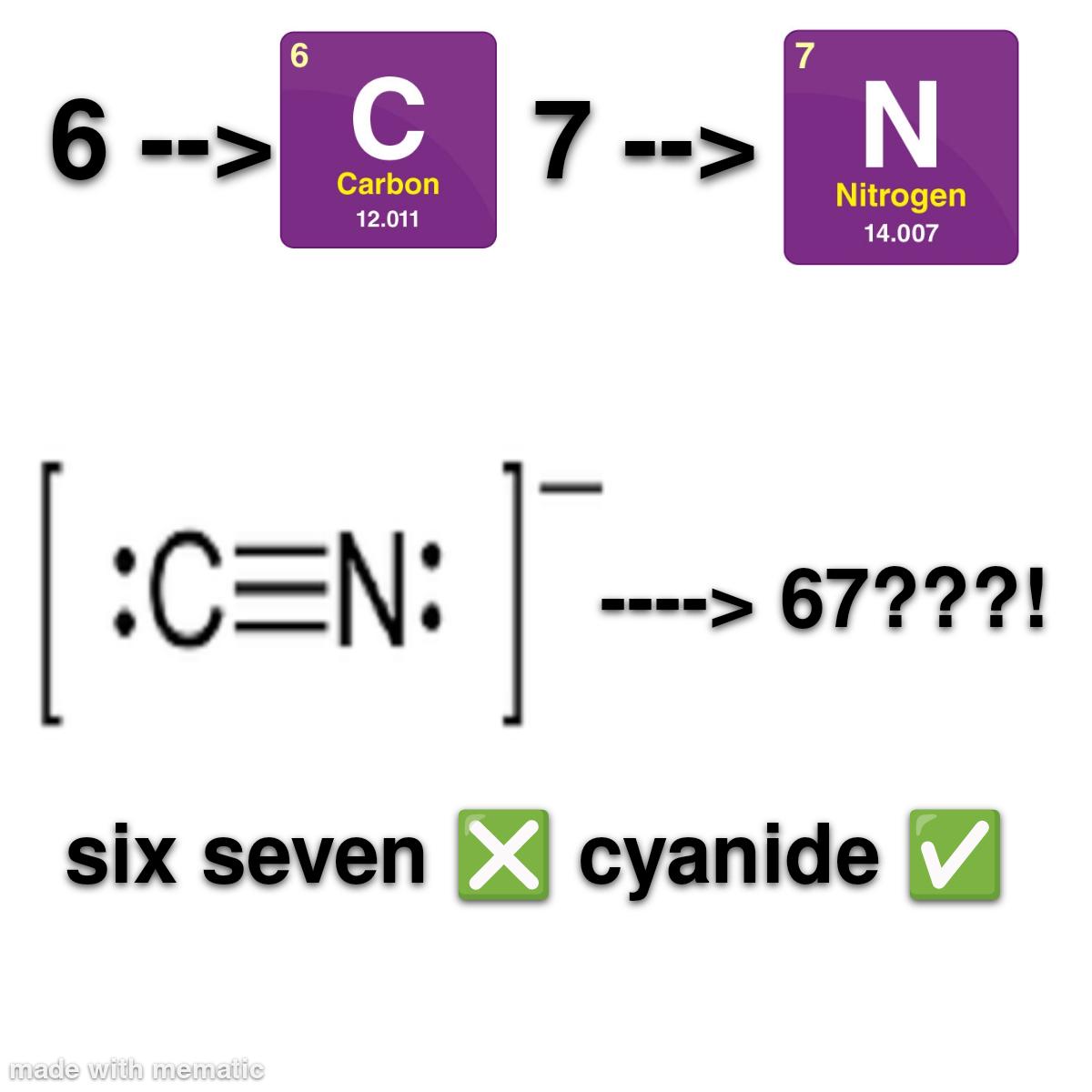
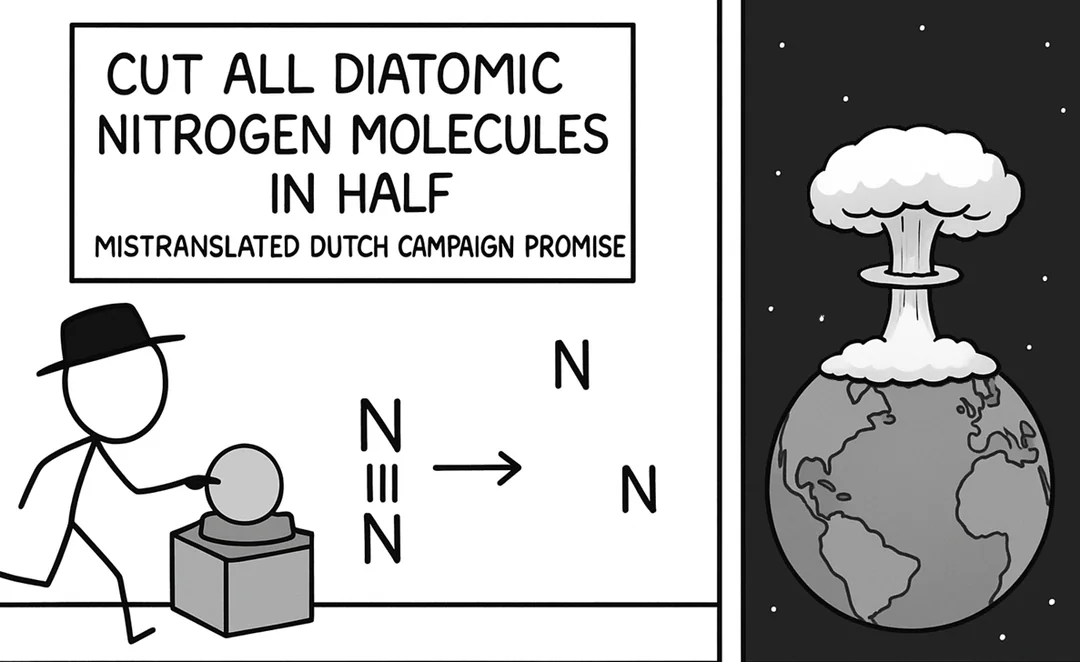
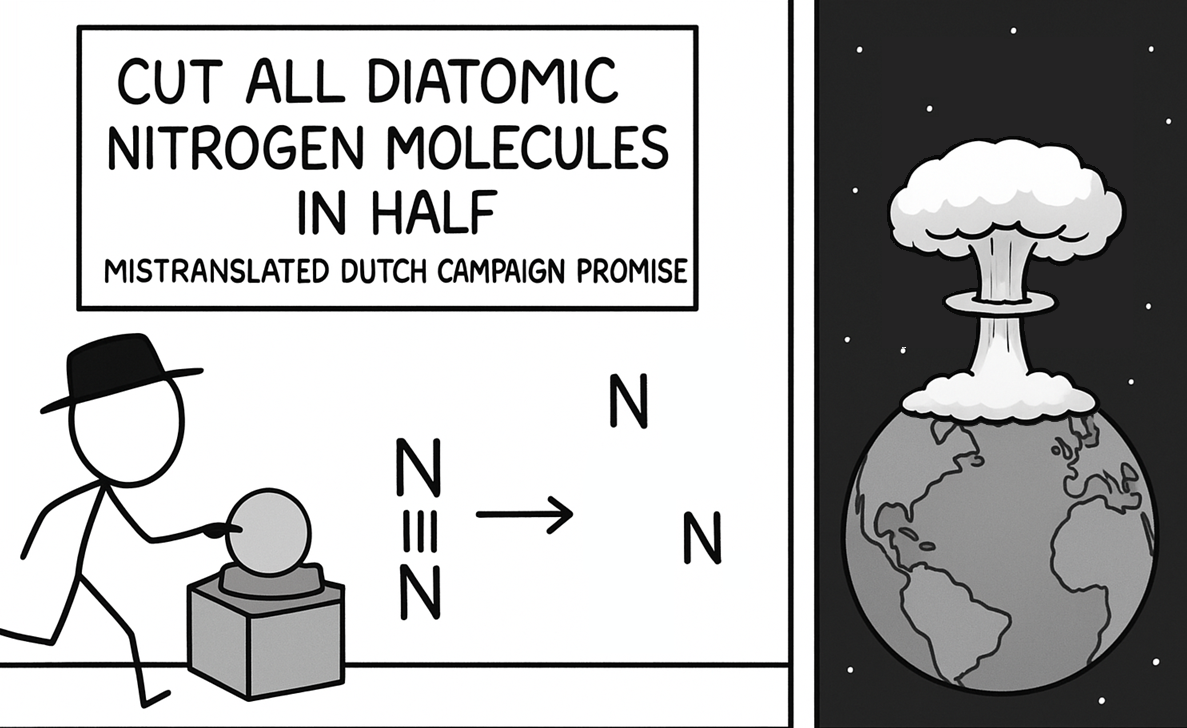
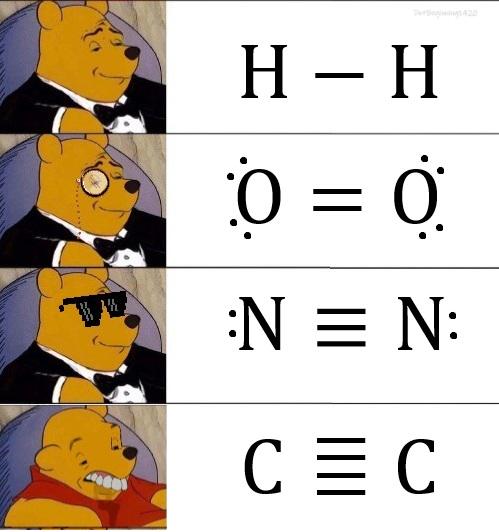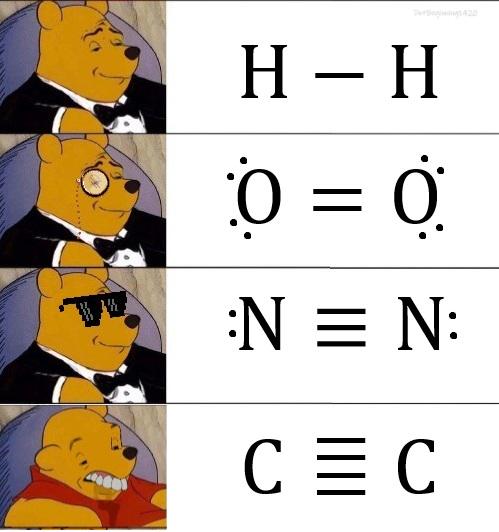The eternal scientific rivalry captured in one perfect meme! Chemists are losing their minds over basic classification ("YOU CAN'T CALL NITROGEN A METAL!") while astrophysicists are just sitting there, unbothered like that confused cat at dinner. Chemists get super territorial about element classifications because that's their whole world. Meanwhile, astrophysicists are dealing with exploding stars, black holes, and the fabric of spacetime itself—they couldn't care less about your periodic table drama! It's the perfect representation of how different scientific disciplines have wildly different priorities. The stuff that makes one field freak out completely flies under the radar in another!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology