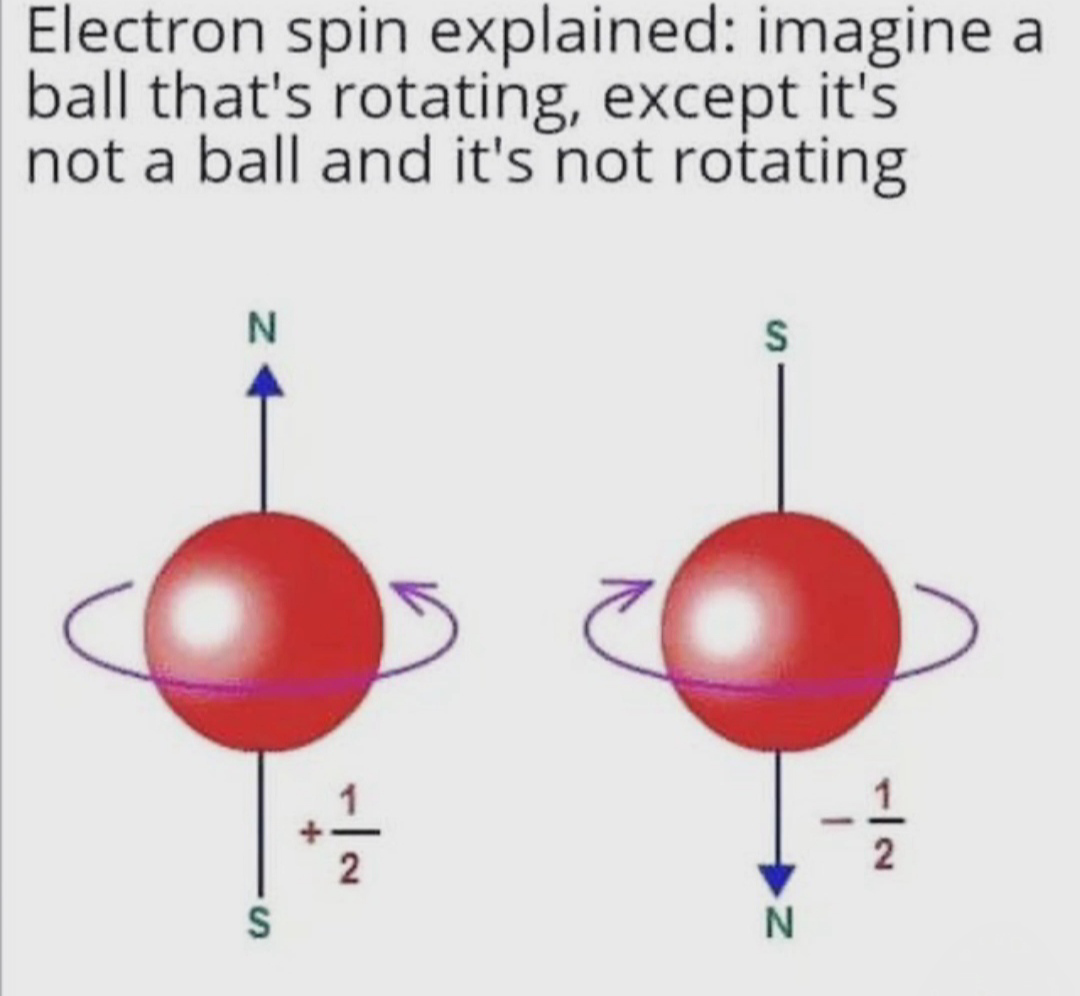
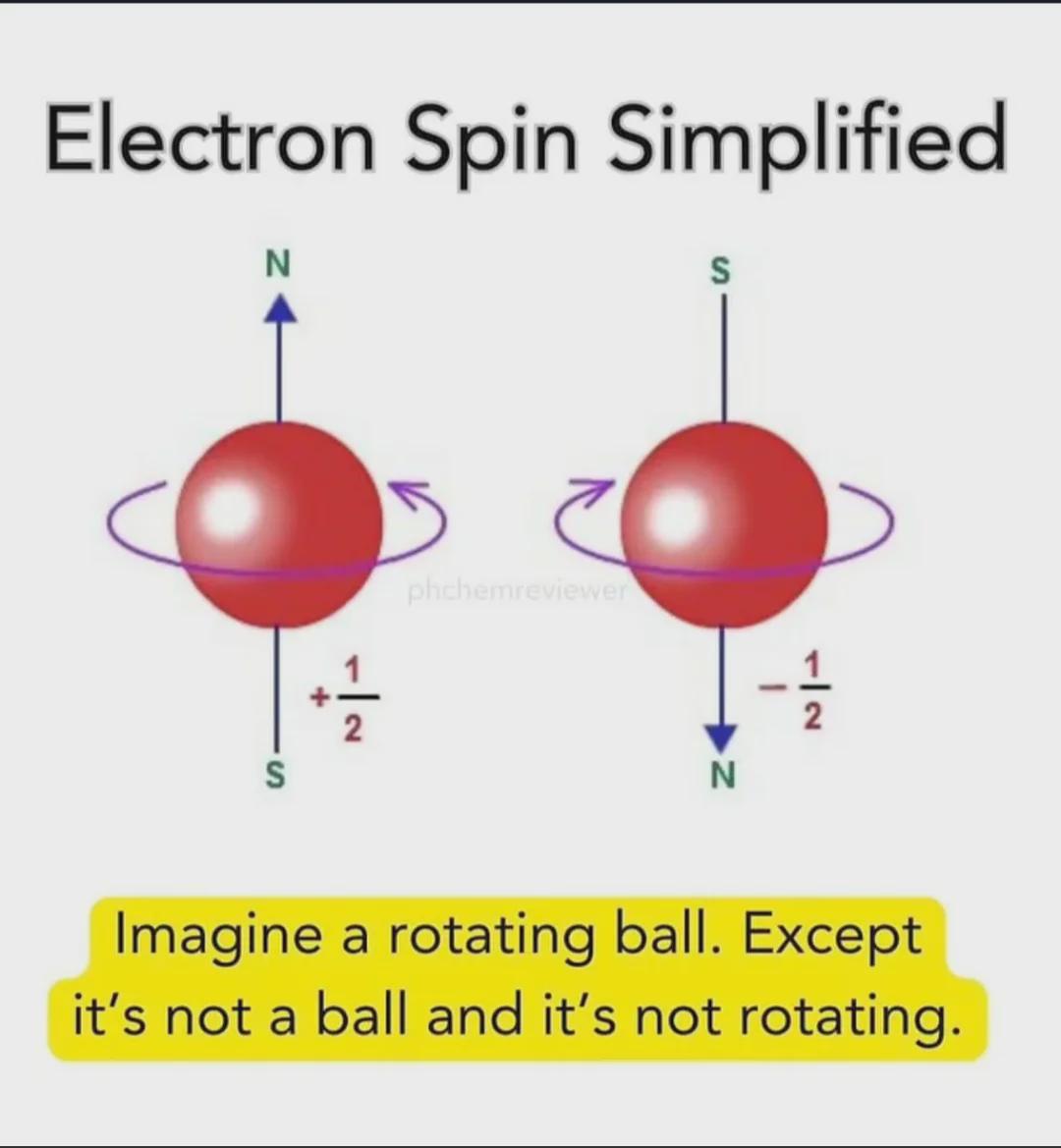
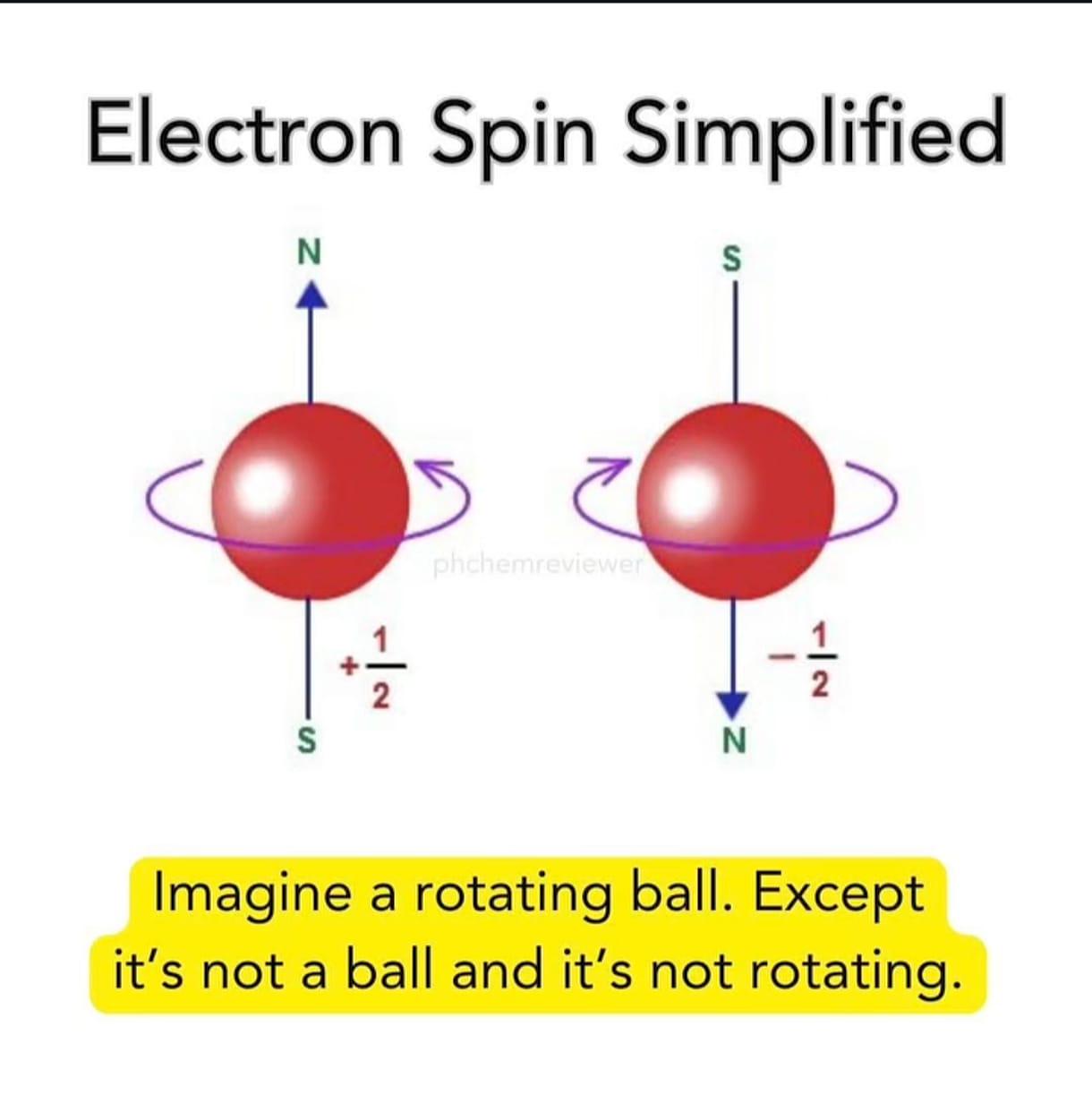
Quantum physics has a special talent for making your brain hurt! The meme perfectly captures how physicists try to explain electron spin to the rest of us mortals. "Imagine a ball that's rotating, except it's not a ball and it's not rotating." Thanks for clearing that up, science! 😂 What makes this hilarious is that electron spin is actually a quantum property with no classical equivalent. Scientists use the rotating ball analogy to help us visualize it, then immediately destroy that visualization by saying "but actually, it's nothing like that." Classic physics move - build a mental model then set it on fire! The +1/2 and -1/2 values shown are the actual quantum spin numbers, and they're literally the best we can do to describe something that exists beyond our everyday experience. Quantum mechanics: where even the explanations need explanations!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology