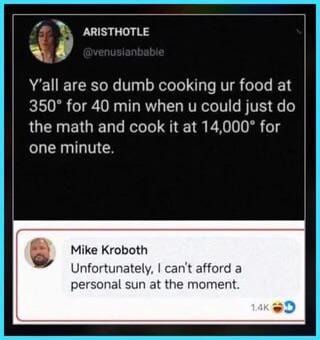
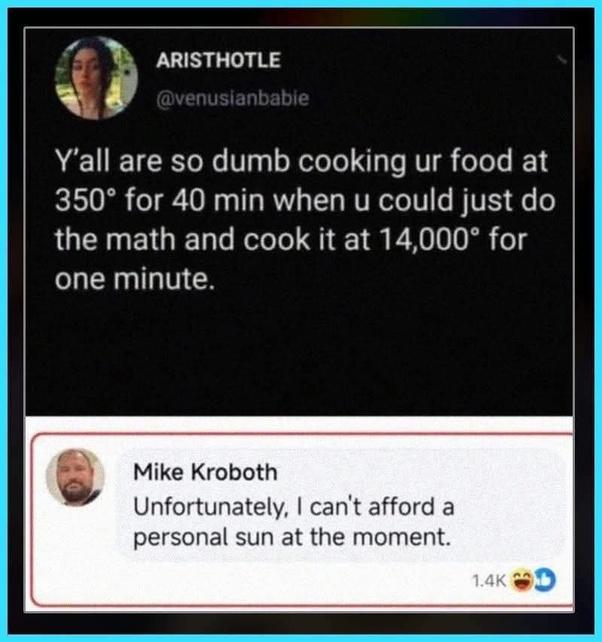
Oh look, someone skipped thermodynamics class to post on social media! The first person thinks they've discovered some revolutionary cooking hack—just crank up the temperature by 40x and reduce the time proportionally. Genius! Except that's how you get a kitchen full of smoke alarms and a visit from your local fire department. Mike's response is pure gold though. The surface temperature of the sun is around 10,000°F (5,500°C), so he's basically saying "Yeah, I'd love to incinerate my dinner with a personal star, but my budget doesn't quite cover astronomical objects this quarter." And to think Aristotle would be proud of this exchange. Two thousand years of scientific progress to arrive at... this.


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology