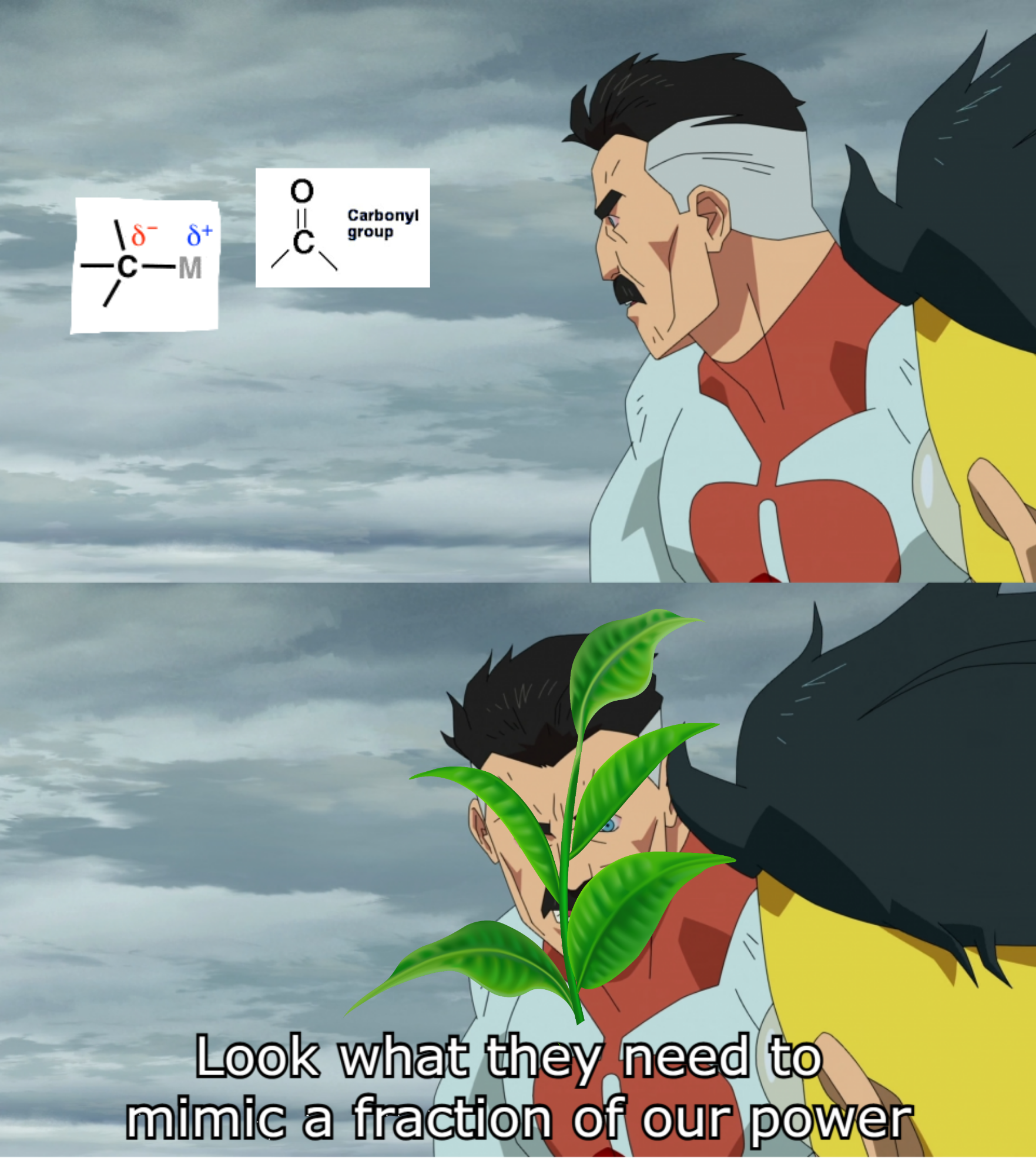
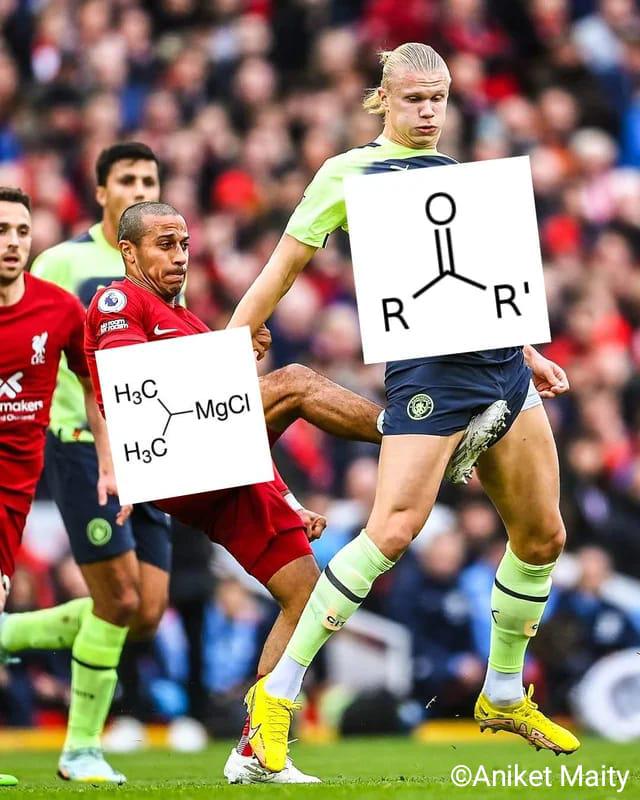
Plants out here flexing on organic chemists like it's nothing! While chemists struggle with complex reagents, catalysts, and precise conditions to form carbon-carbon bonds, plants are just casually performing photosynthesis, building glucose molecules from CO 2 like "no big deal." The carbonyl group and organometallic reagent shown are the chemist's tools requiring fancy labs and hazardous chemicals, while plants need only sunlight, water, and their chlorophyll superpowers. Next time you're sweating over a Grignard reaction, remember there's a houseplant somewhere doing more impressive carbon chemistry while looking fabulous.


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology