How many scientists does it take to change a light bulb? None, they're still writing the grant proposal.
Ad iRobot Roomba j7+
Ignore it for 60 days, just like code warnings

Purchase this and help us pay for the cloud storage to back up all those genome sequence files. ☁️

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
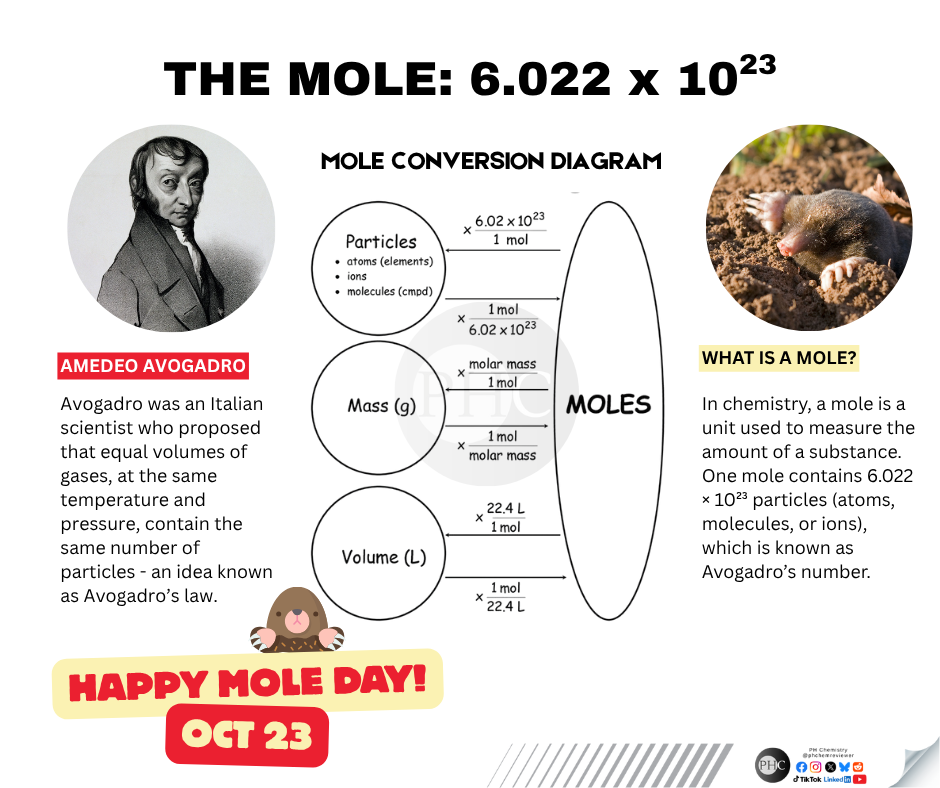
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology