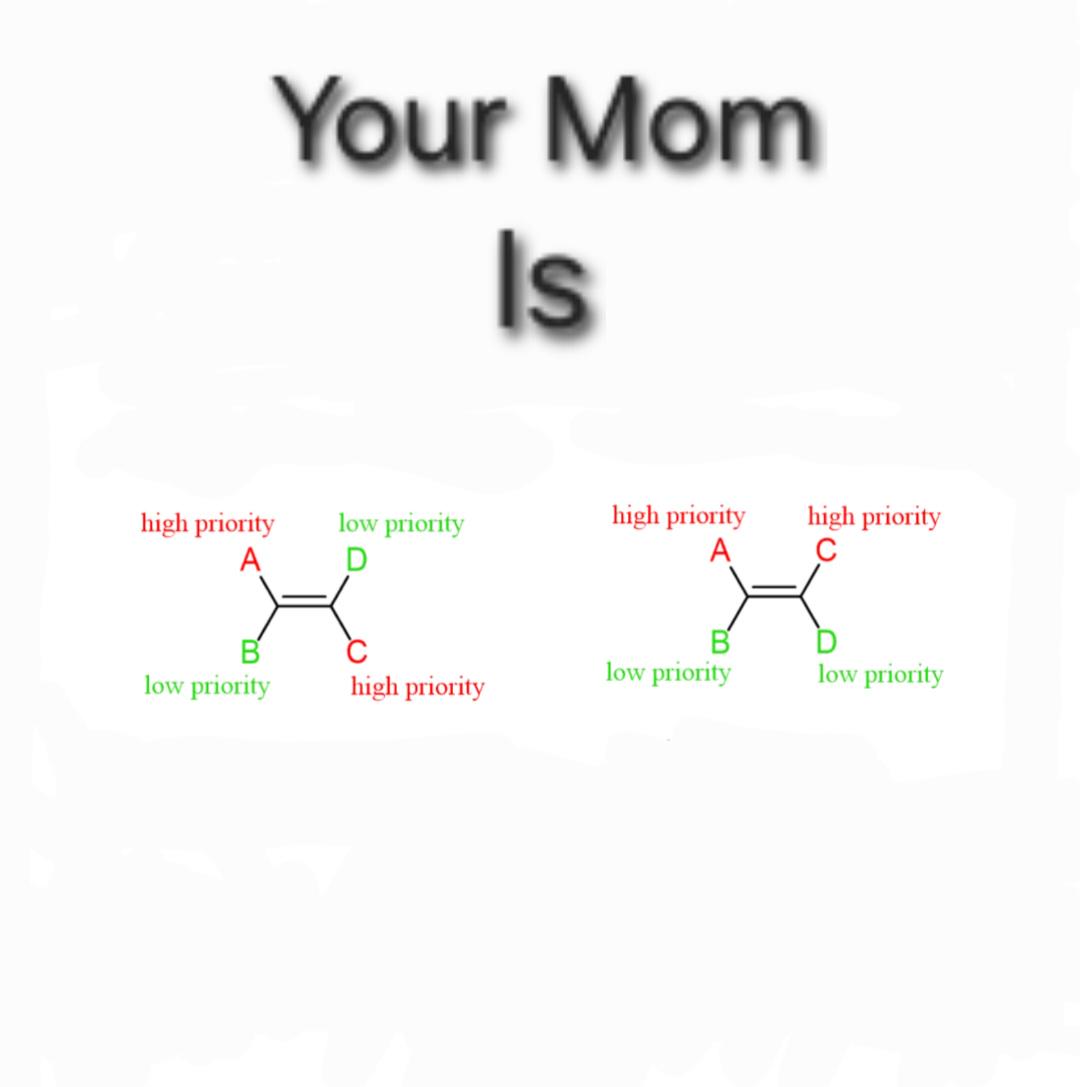
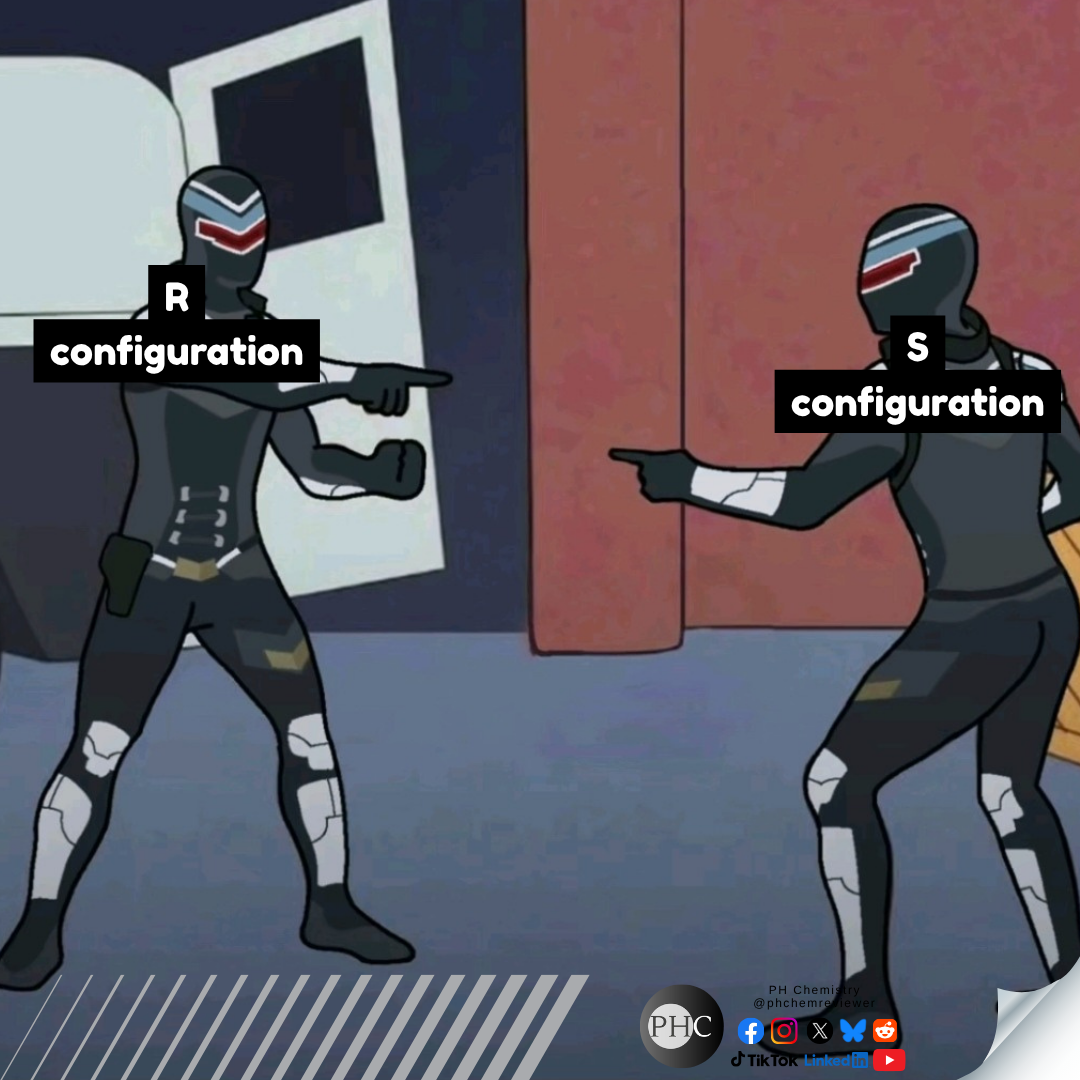
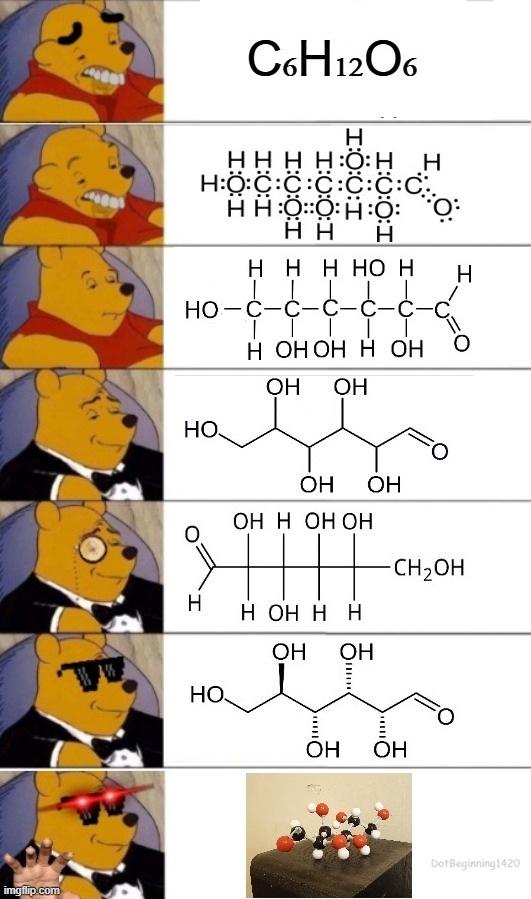
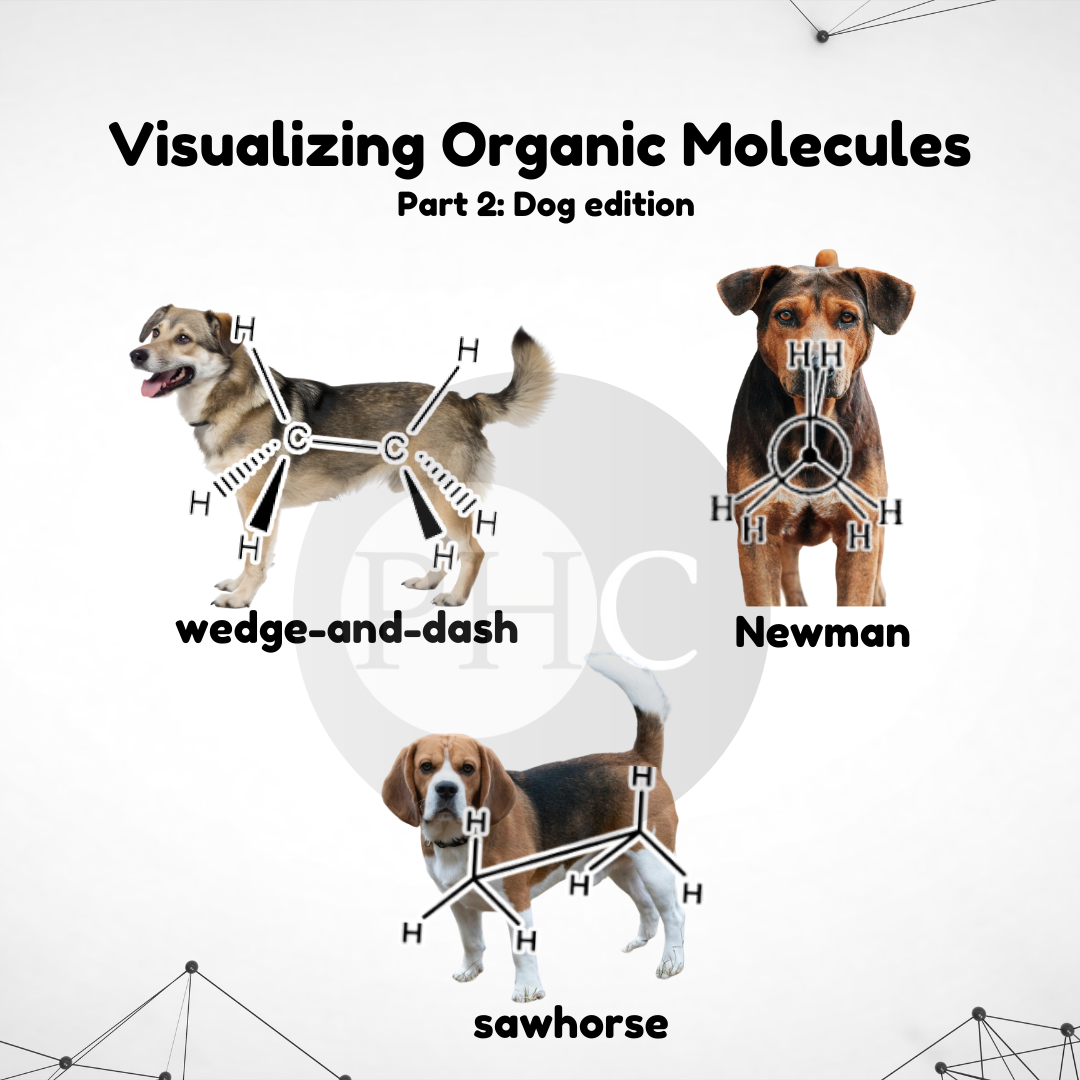
Oh, the sweet summer child who thinks organic chemistry is "a piece of cake." That moment when reality crashes harder than a failed column chromatography! Organic chem starts with friendly-looking carbon chains and ends with you drawing reaction mechanisms at 3 AM while questioning your life choices. The betrayal hits when you realize those "simple" hexagons actually represent a labyrinth of stereochemistry, nucleophilic substitutions, and synthesis pathways that make Game of Thrones plot twists look predictable. Trust me, the only thing organic about this experience is the pure, organic suffering.


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology