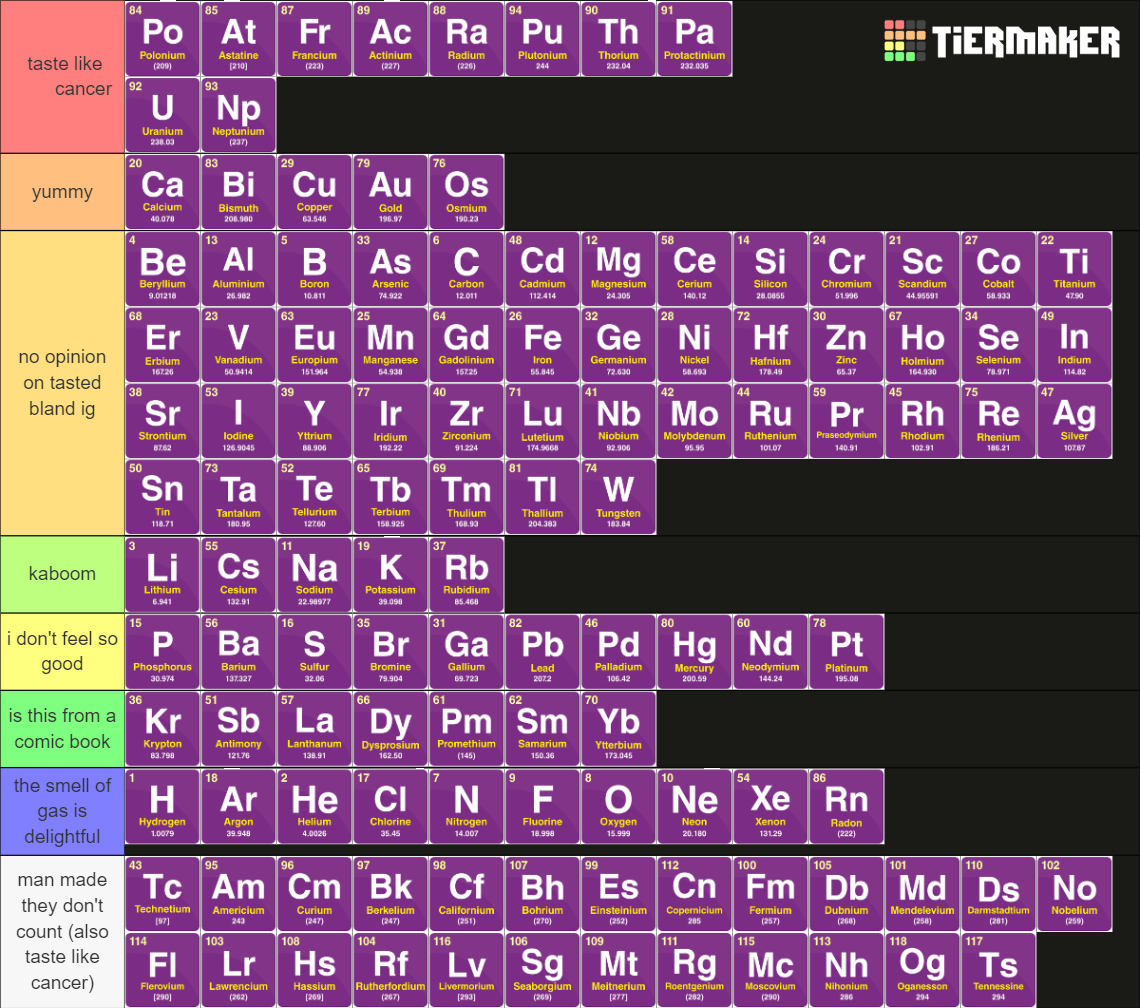
The periodic table's most dramatic relationship status update! Alkali metals (top) are desperate to give away their electrons, practically flashing them like a sketchy dude with a trench coat. Noble gases (middle) are the snobs of chemistry, rejecting electrons with a hard "no thanks, I'm complete." Meanwhile, halogens (bottom) are the electron-hungry vultures, ready to mug you for that extra electron to complete their outer shell. It's like watching three different dating strategies at the atomic nightclub—desperate flirting, playing hard to get, and straight-up electron theft. Chemistry isn't just a science; it's a soap opera where the drama revolves around who's sharing electrons with whom!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology