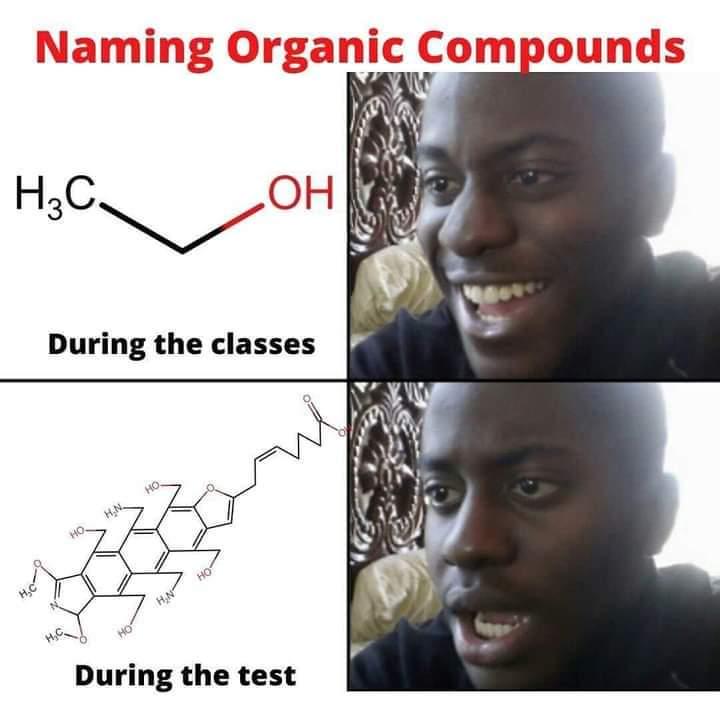
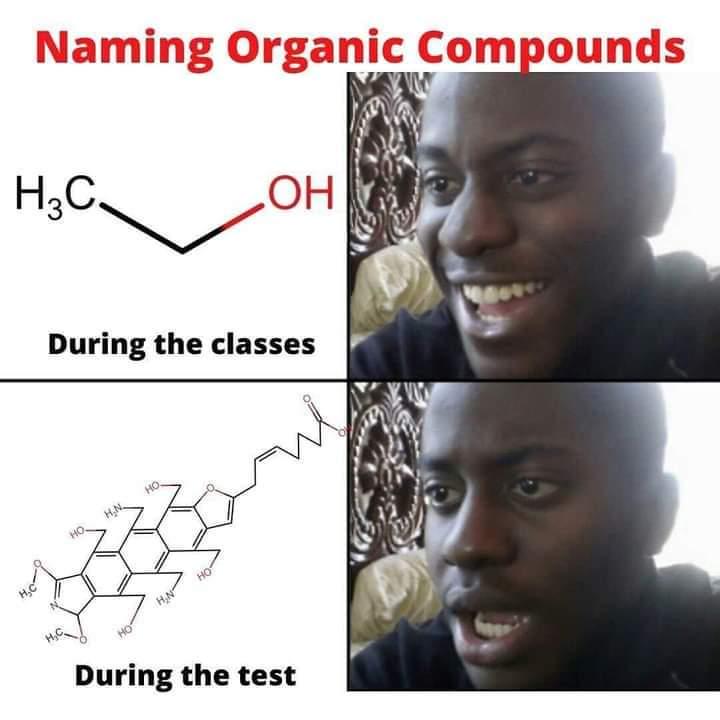
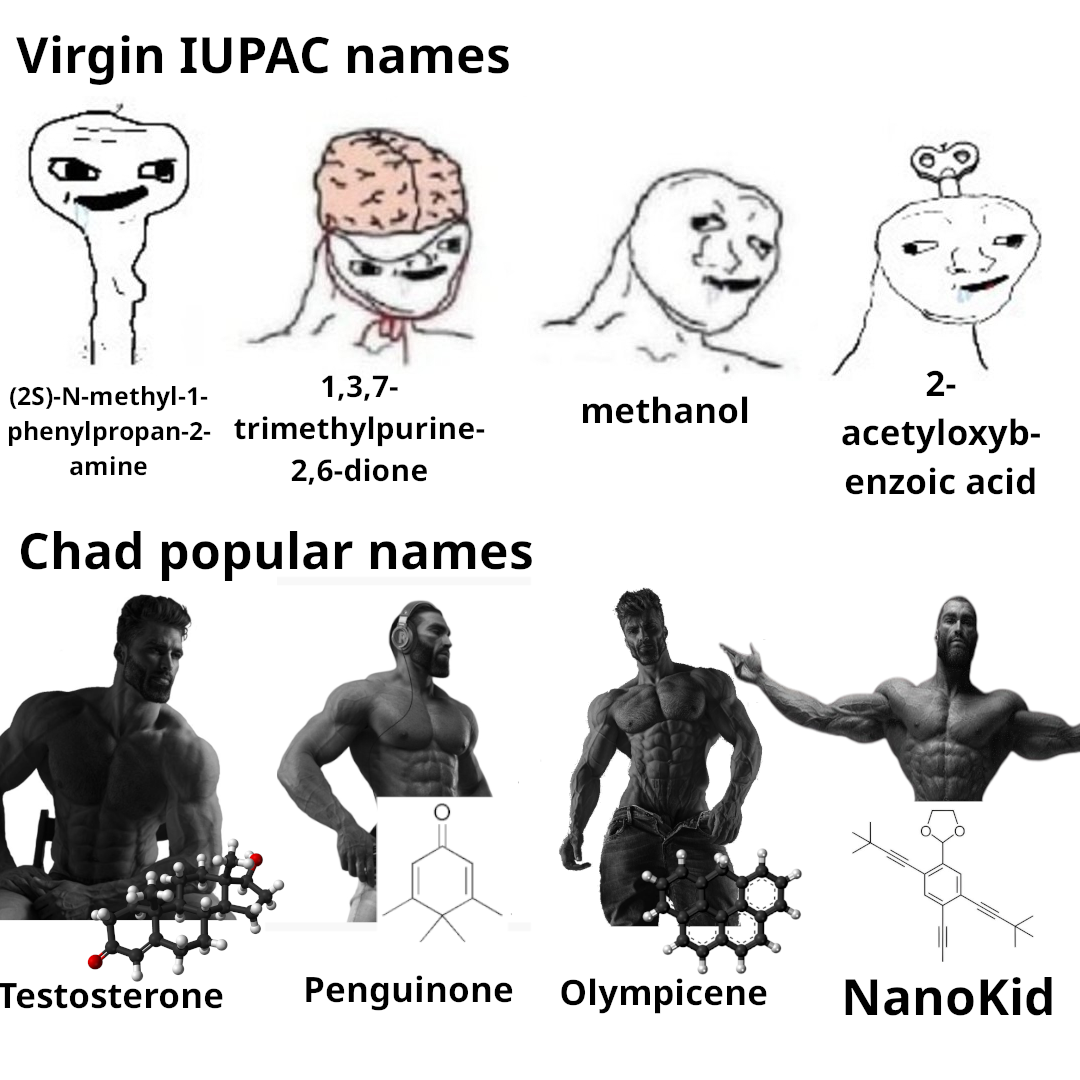
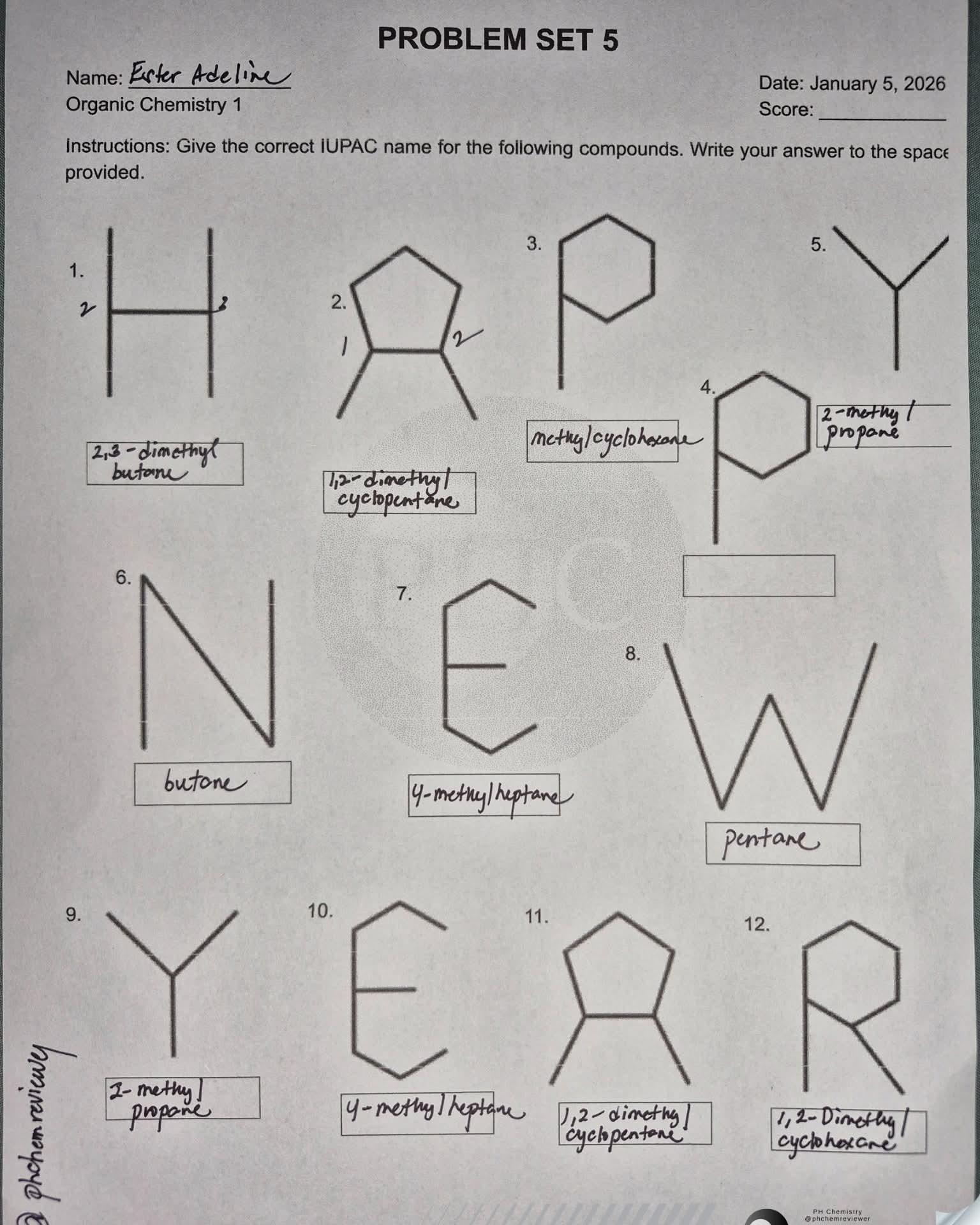
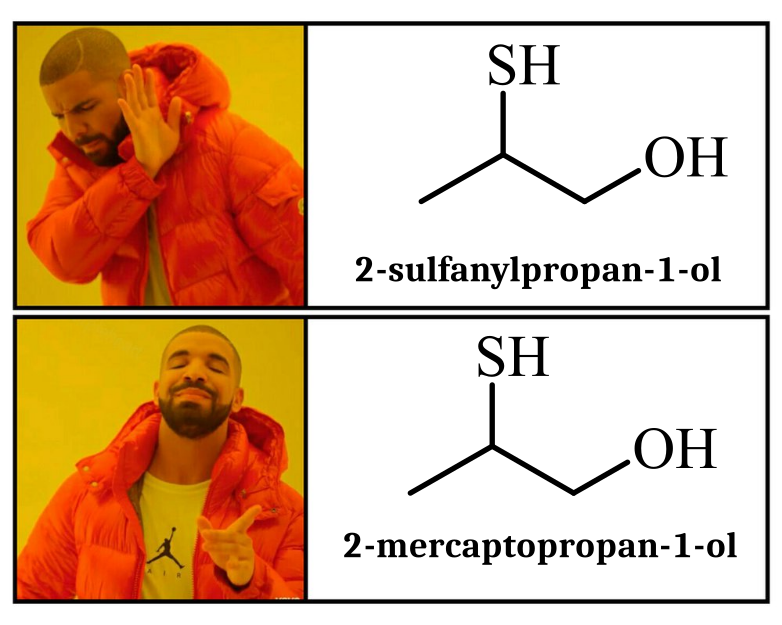
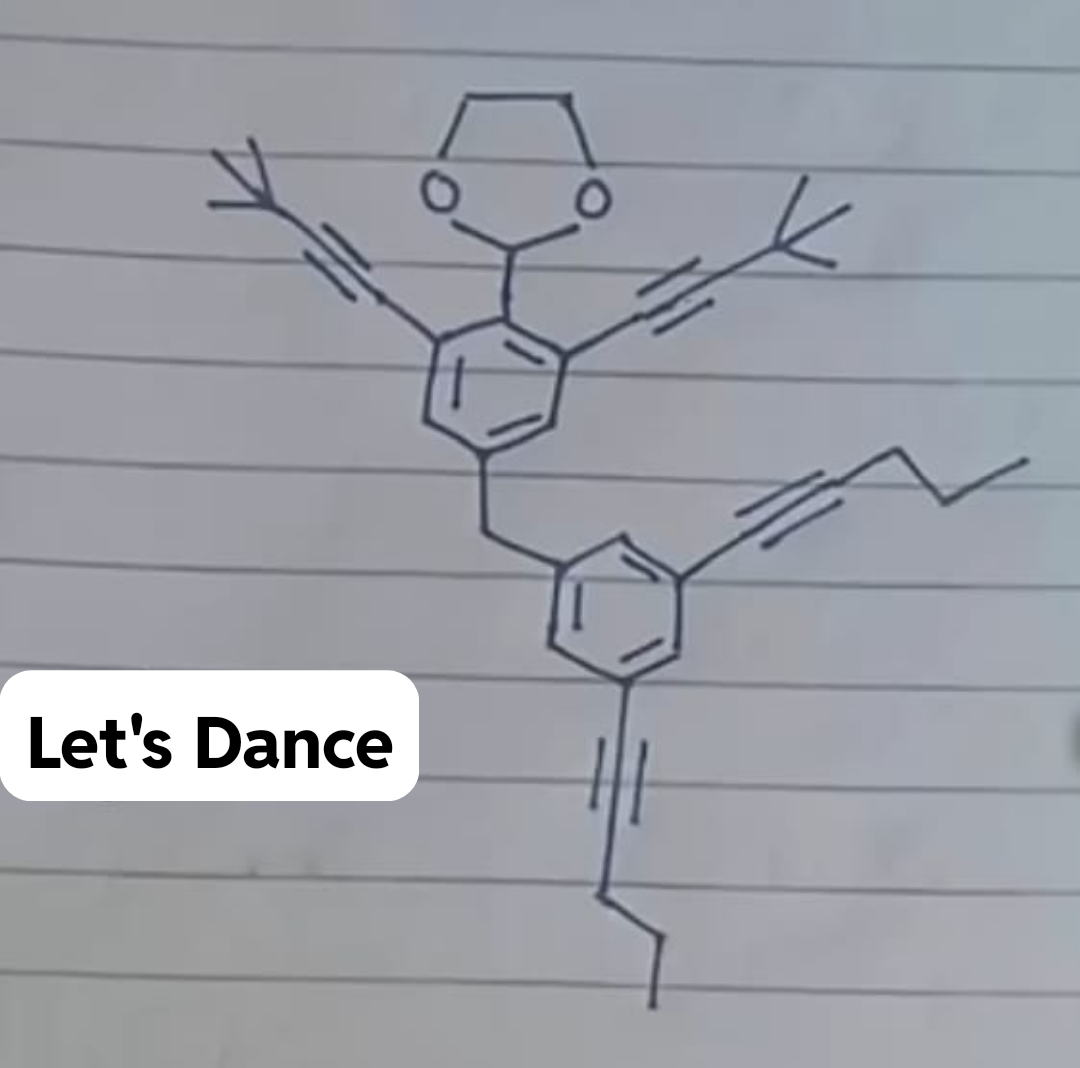
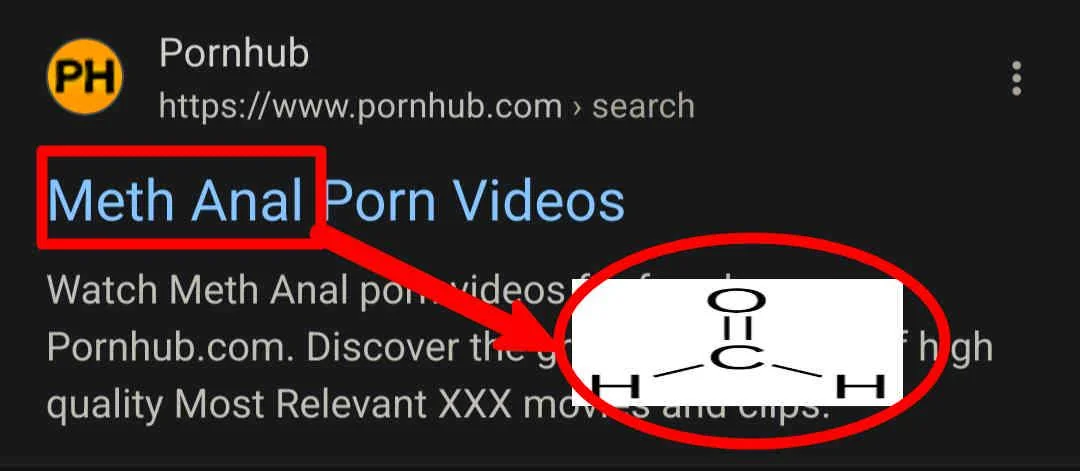
Chemistry nerds evolving into their final form! The meme shows how we start with simple "cholesterol" (boring, casual), level up to "cholest-5-en-3β-ol" (now we're talking!), and finally achieve chemical enlightenment with that monstrosity of numbers and symbols at the bottom. It's like watching a Pokémon evolution, but for people who get excited about naming conventions! The systematic IUPAC name is basically the chemical equivalent of giving someone your full address including GPS coordinates when they just asked where you live. Pure chemistry flex. The longer the name, the more powerful the chemist!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology