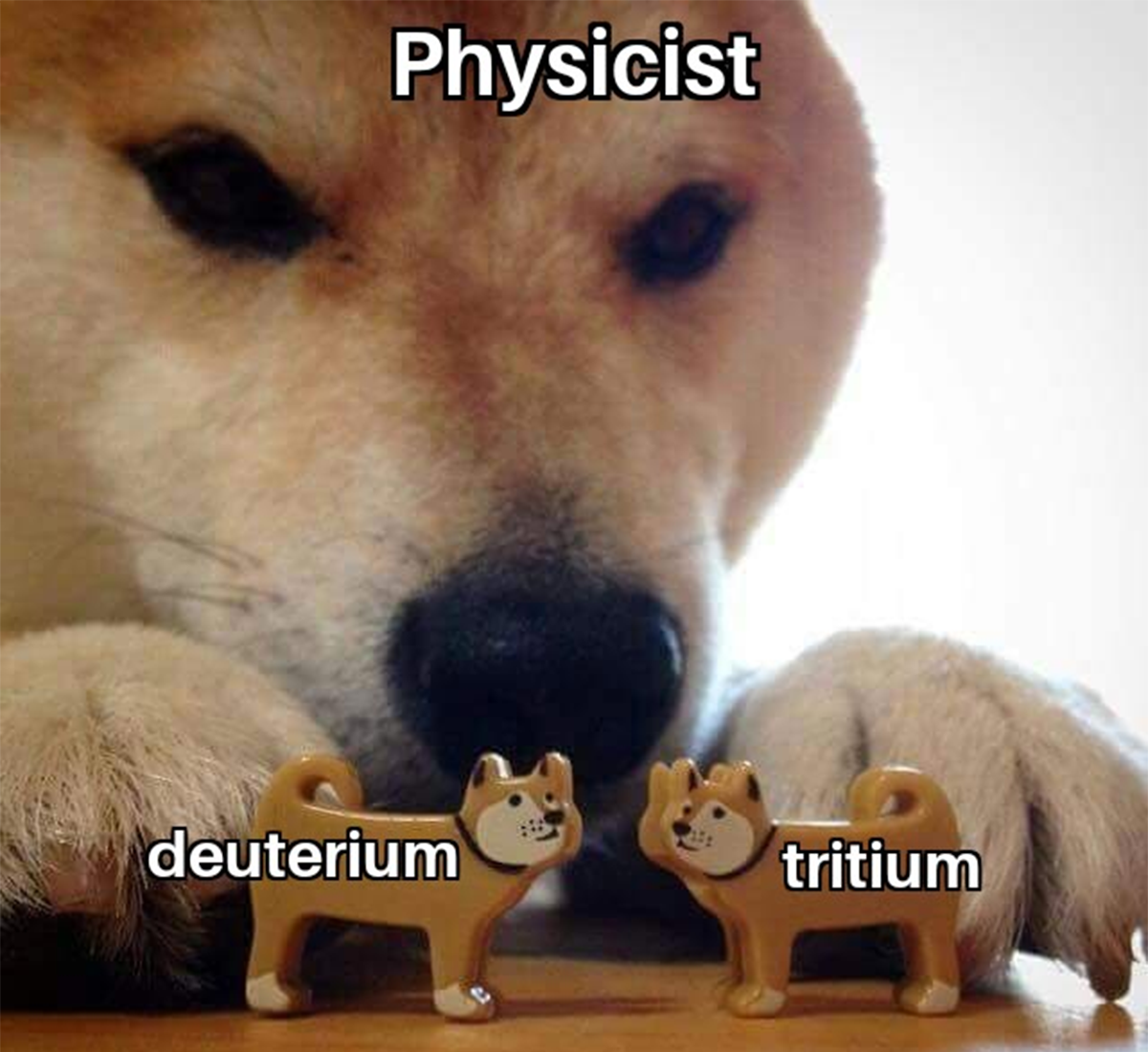
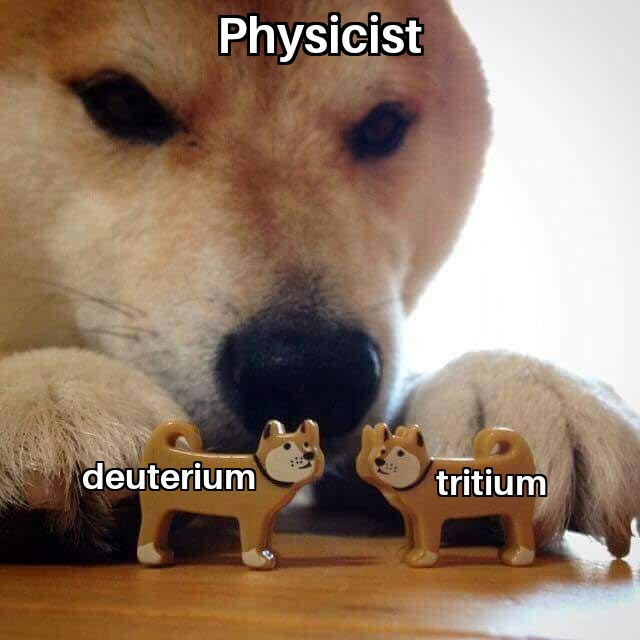
Ever had that existential crisis when you discover certain atomic masses are just doomed to be unstable? Nuclear physics doesn't care about your feelings! Those specific nuclides (5, 8, 147, 151) are all radioactive because their nuclear configurations are fundamentally unstable - Mother Nature's way of saying "this arrangement just won't work long-term." It's like trying to balance a pencil on its tip - theoretically possible, but physics is gonna physics. The universe has trust issues with these particular atomic arrangements!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology