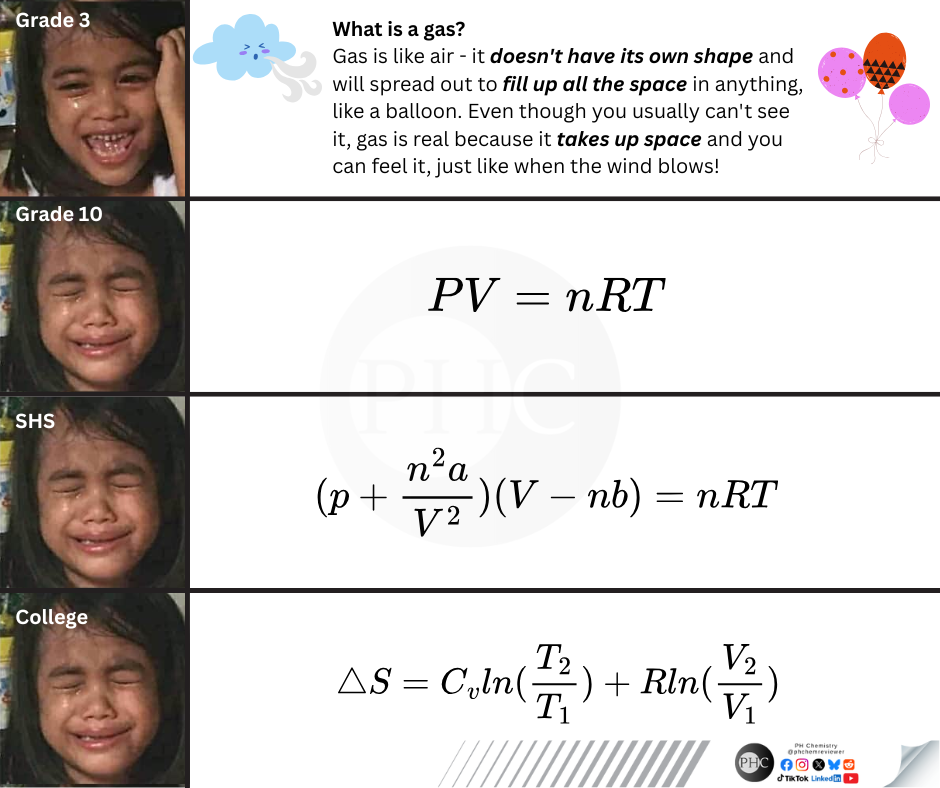
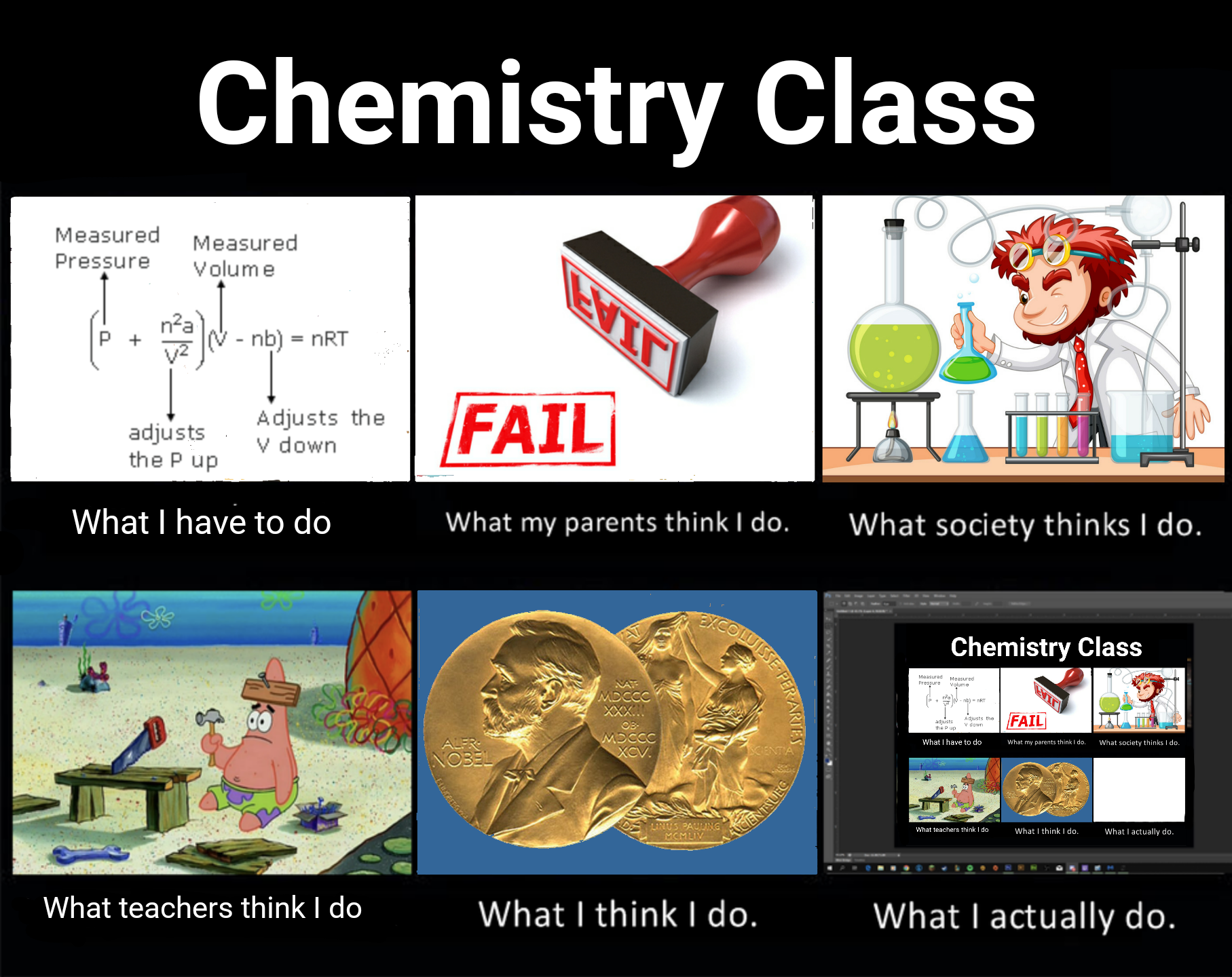
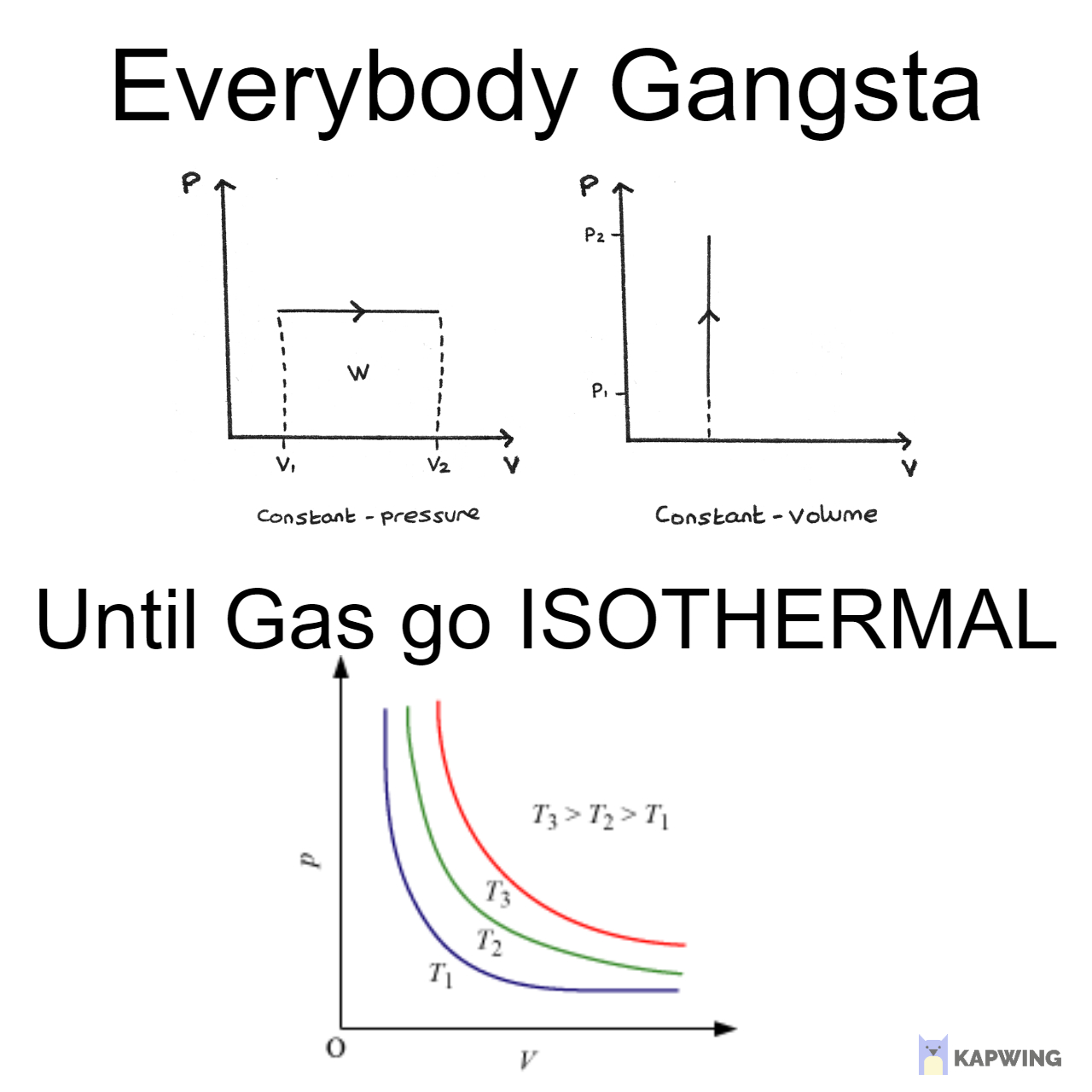
The educational journey of understanding gases is basically the story of your soul slowly leaving your body! Starting with "gas is like air" and cute balloon examples, you're suddenly thrown into the deep end with PV = nRT (ideal gas law). Just when you think you've got it, high school hits you with van der Waals equation that's like "actually, gases aren't ideal, SURPRISE!" Then college delivers the final blow with entropy equations that make you question not just gases but your entire existence. The progression of facial expressions says it all - from innocent joy to pure existential pain. The universal language of thermodynamic suffering!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology