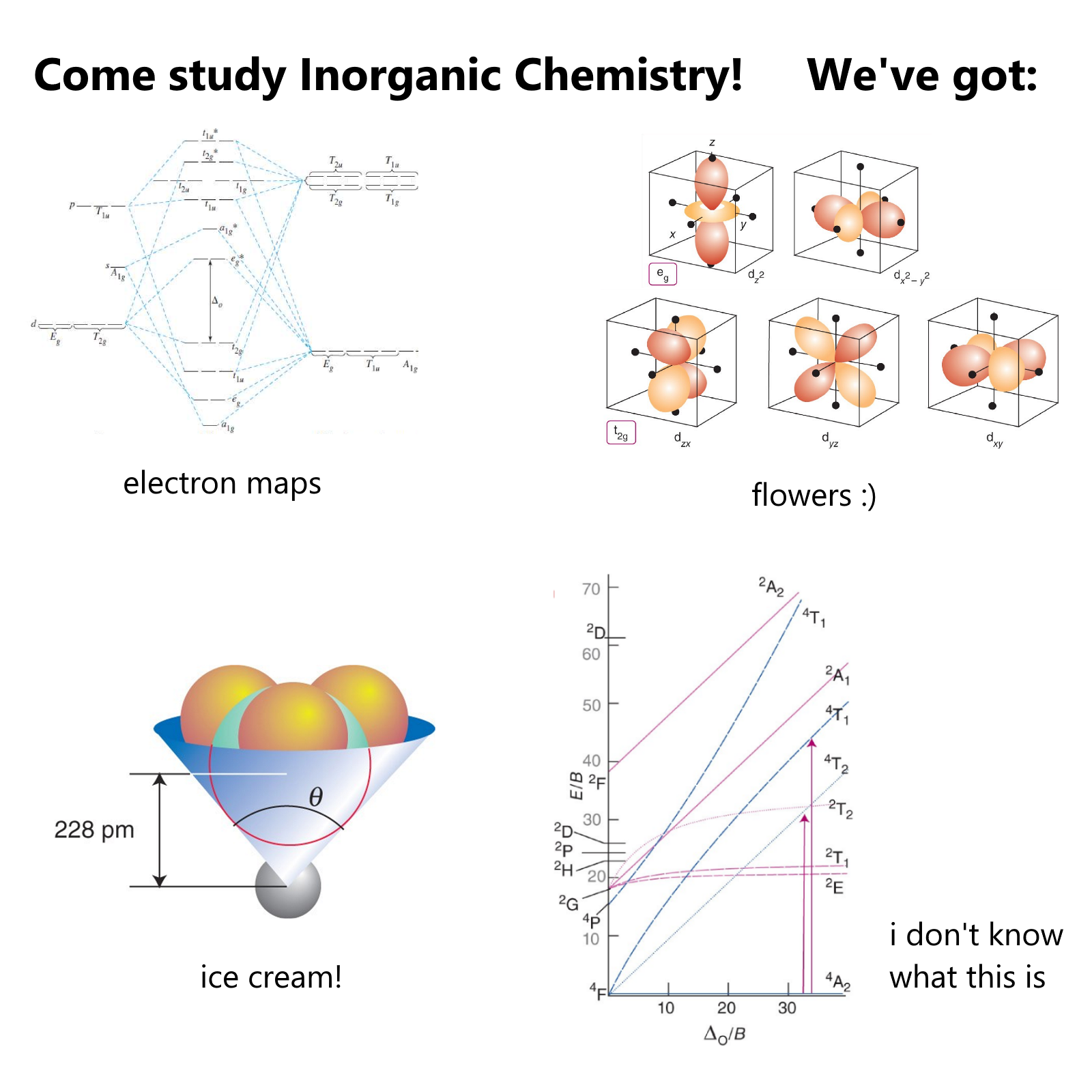
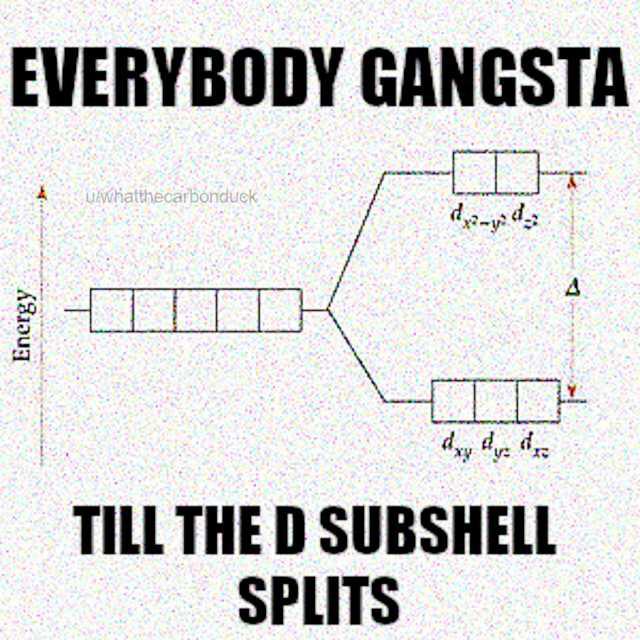
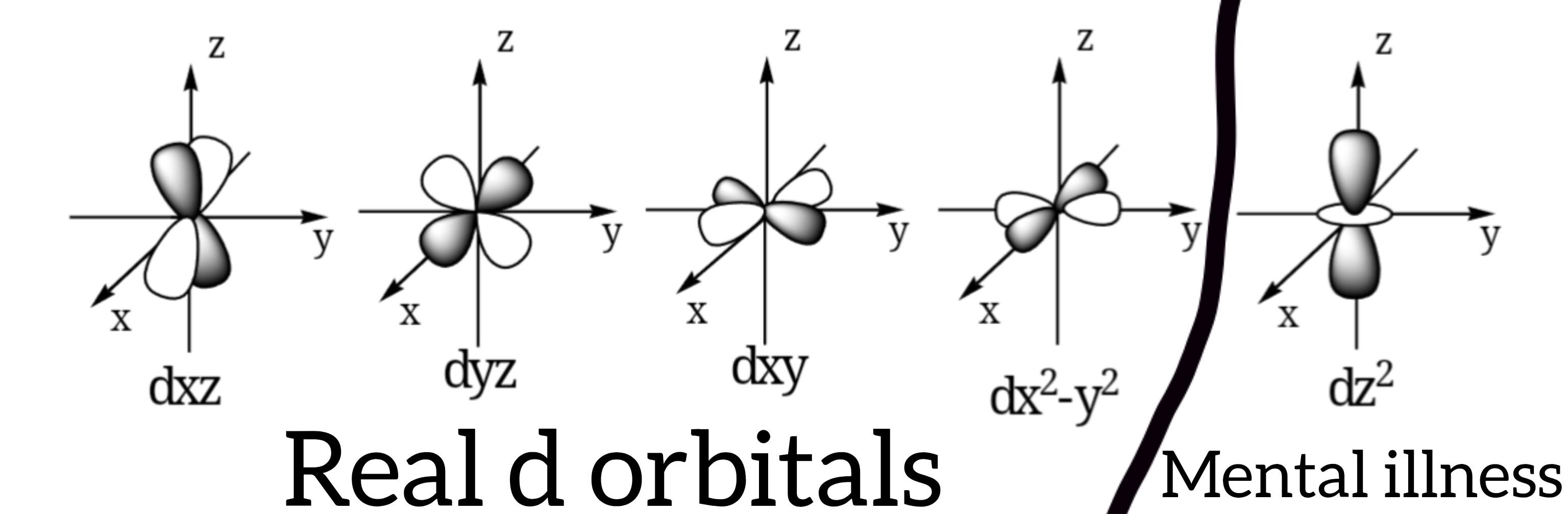
Chemistry students entering their first inorganic class: "I've mastered covalent and ionic bonding!" *Yoda appears* "There is another... and another... and five more after that." Just when you think you've got chemical bonding figured out, metal complexes show up with their d-orbitals, ligand field theory, and molecular orbital diagrams that make your brain hurt. Drawing those full MO diagrams isn't just homework—it's practically a spiritual journey that somehow becomes oddly satisfying once you get the hang of it. Like Sudoku, but with electrons that refuse to behave normally!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology