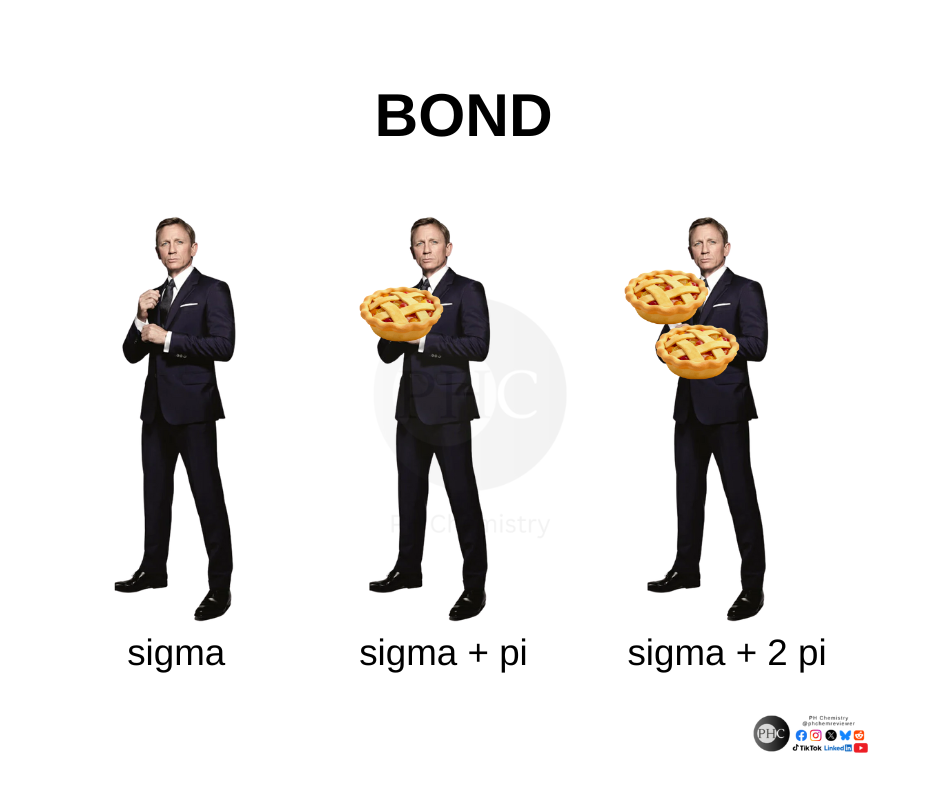
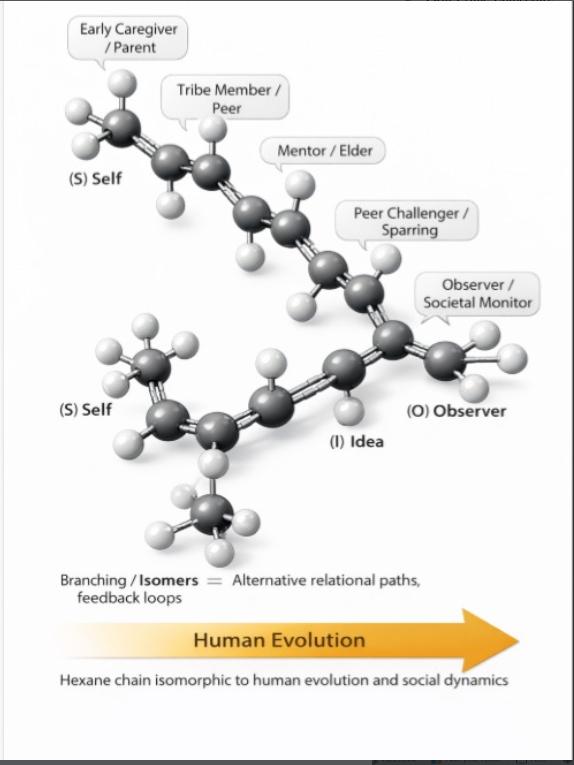
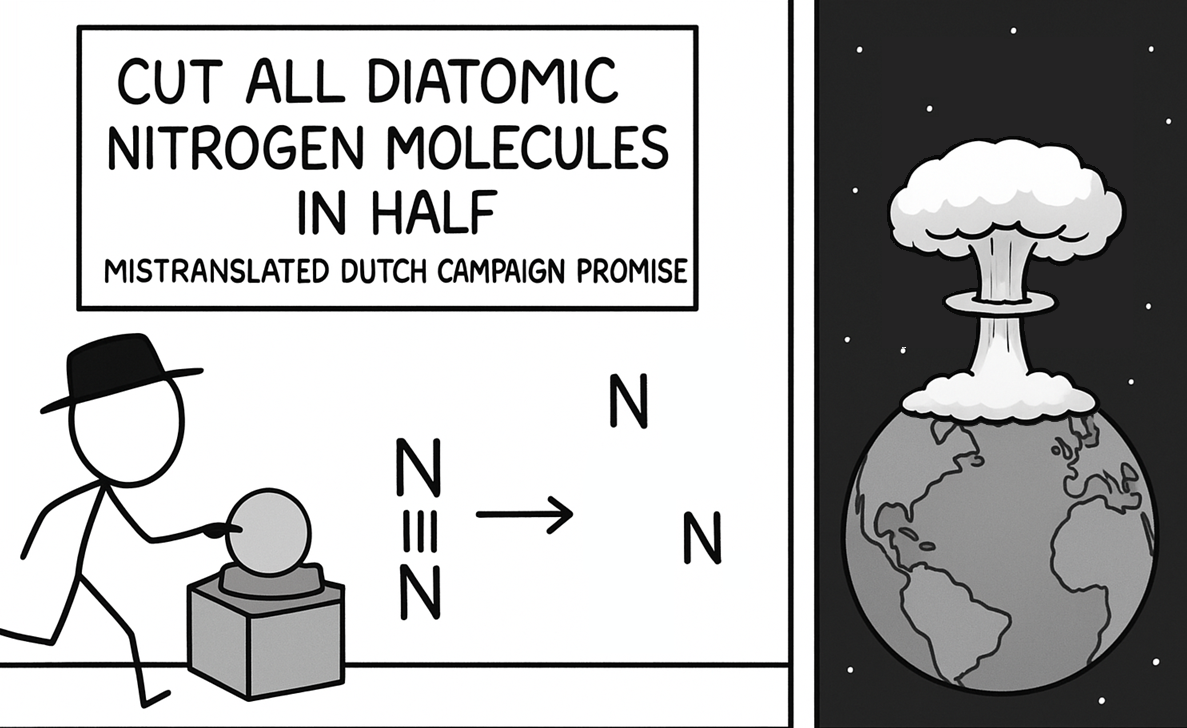
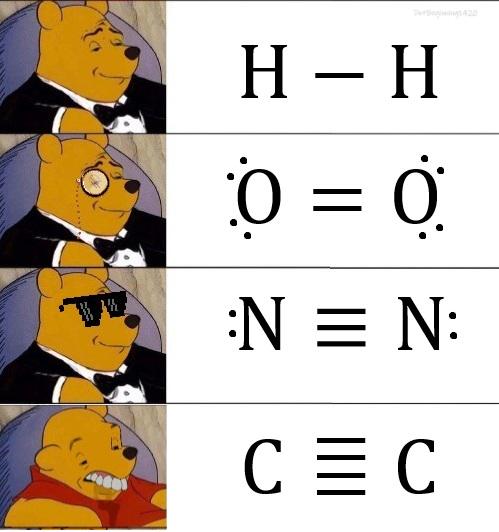Turning relationship advice into molecular wisdom! Just like that toxic ex who wouldn't let go, some covalent bonds hang on for dear life with their electron-sharing death grip. Meanwhile, hydrogen bonds are over here like "let's keep things casual" with their weaker intermolecular forces. 💔⚗️ The beauty of "dissociation kinetics" is just fancy science talk for "knowing when to break up." Even molecules understand that sometimes it's better to split apart than stay in an energetically unfavorable arrangement! Next time someone gives you the "it's not you, it's me" speech, just tell them you respect their dissociation constant. It's thermodynamically inevitable!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology