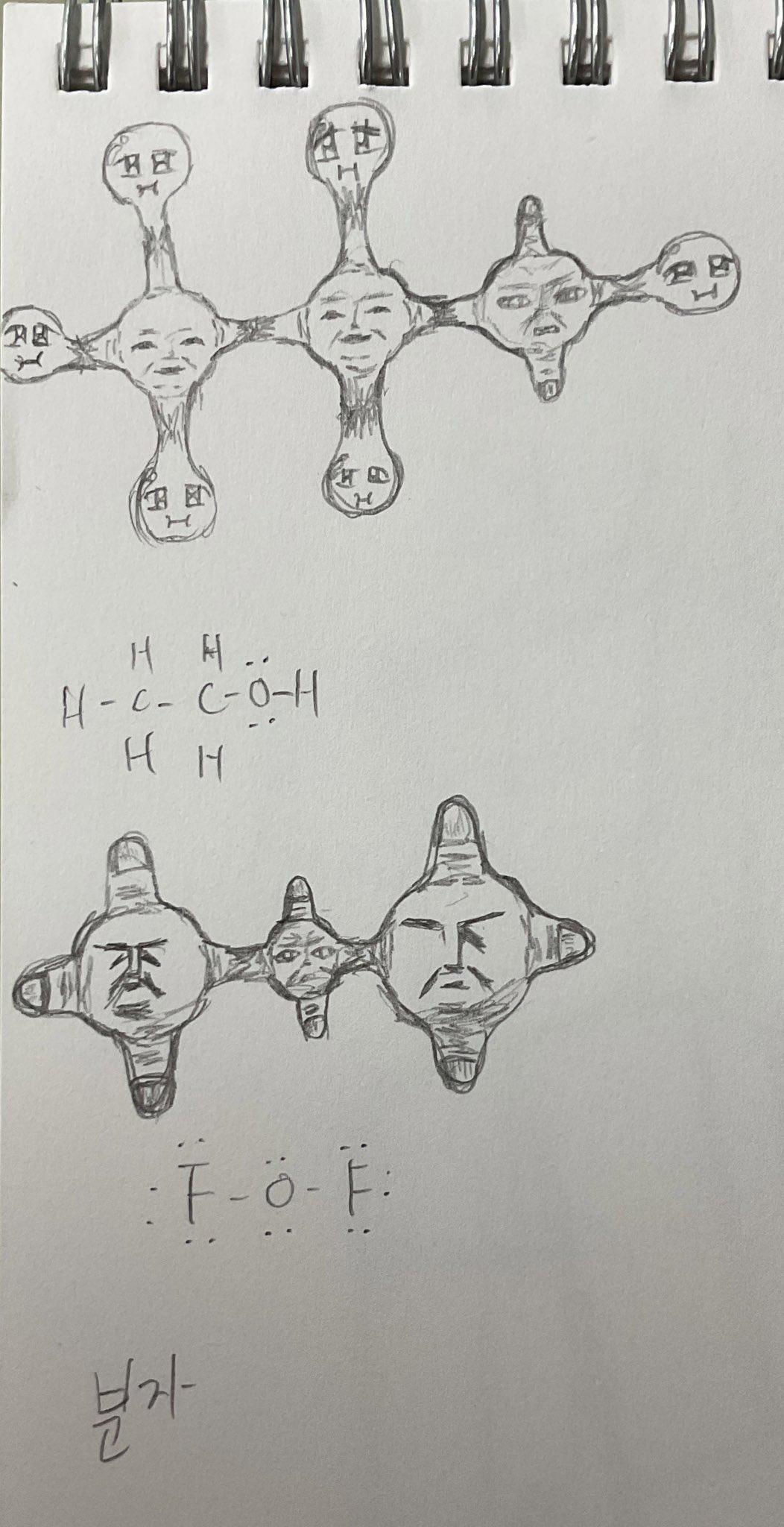
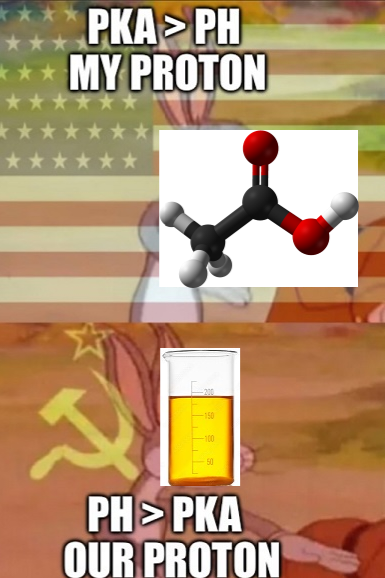
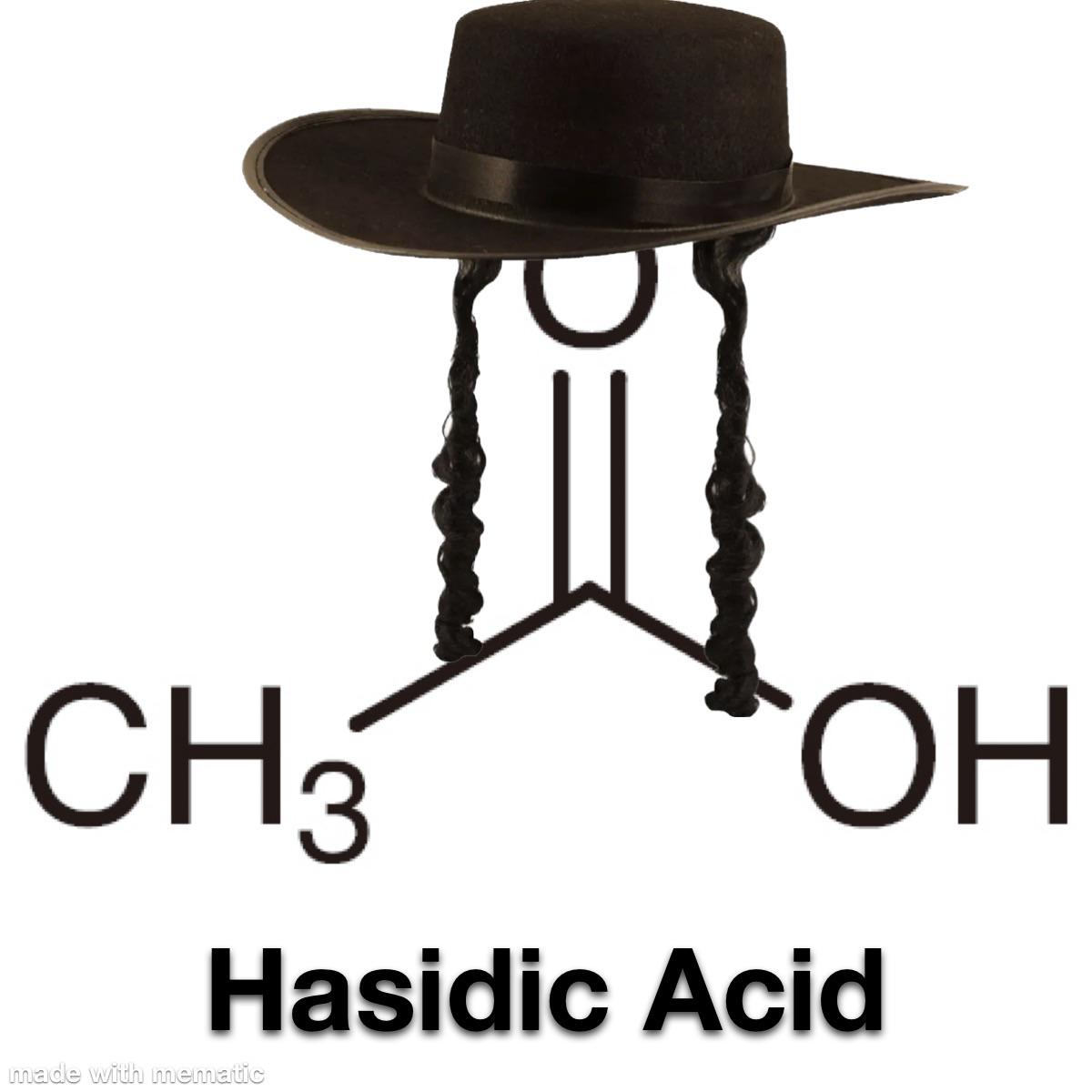
Behold! The human body - nature's most sophisticated biochemical brewery! When you drink alcohol (ethanol), your liver goes into mad scientist mode, frantically converting it to acetic acid. It's literally transforming your weekend fun juice into the same stuff that makes vinegar sour! Your skeleton isn't just supporting you through life's challenges - it's also supporting your body's chemical vendetta against your poor life choices! Next time you're hungover, remember: your bones aren't aching, they're just disappointed in your chemistry experiment gone wrong!


 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology