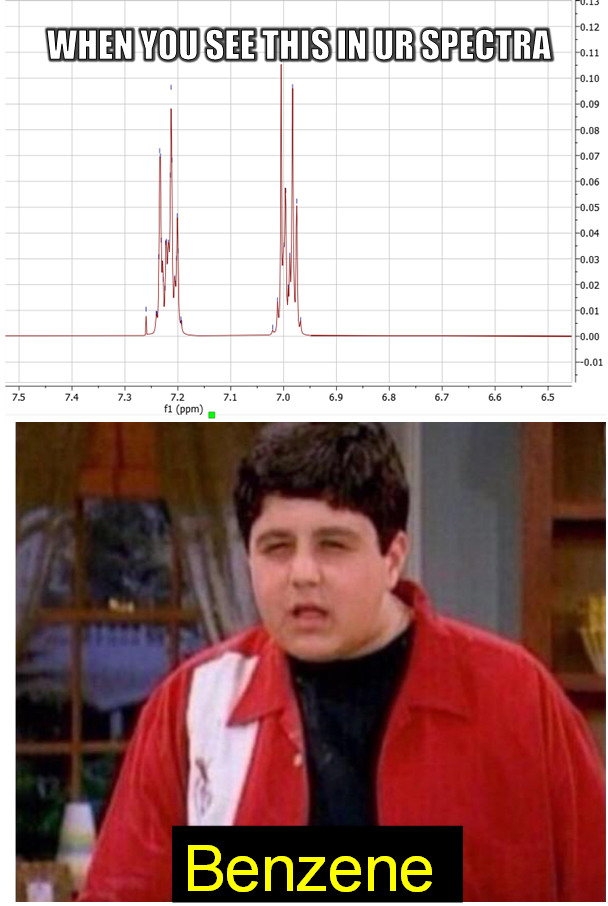
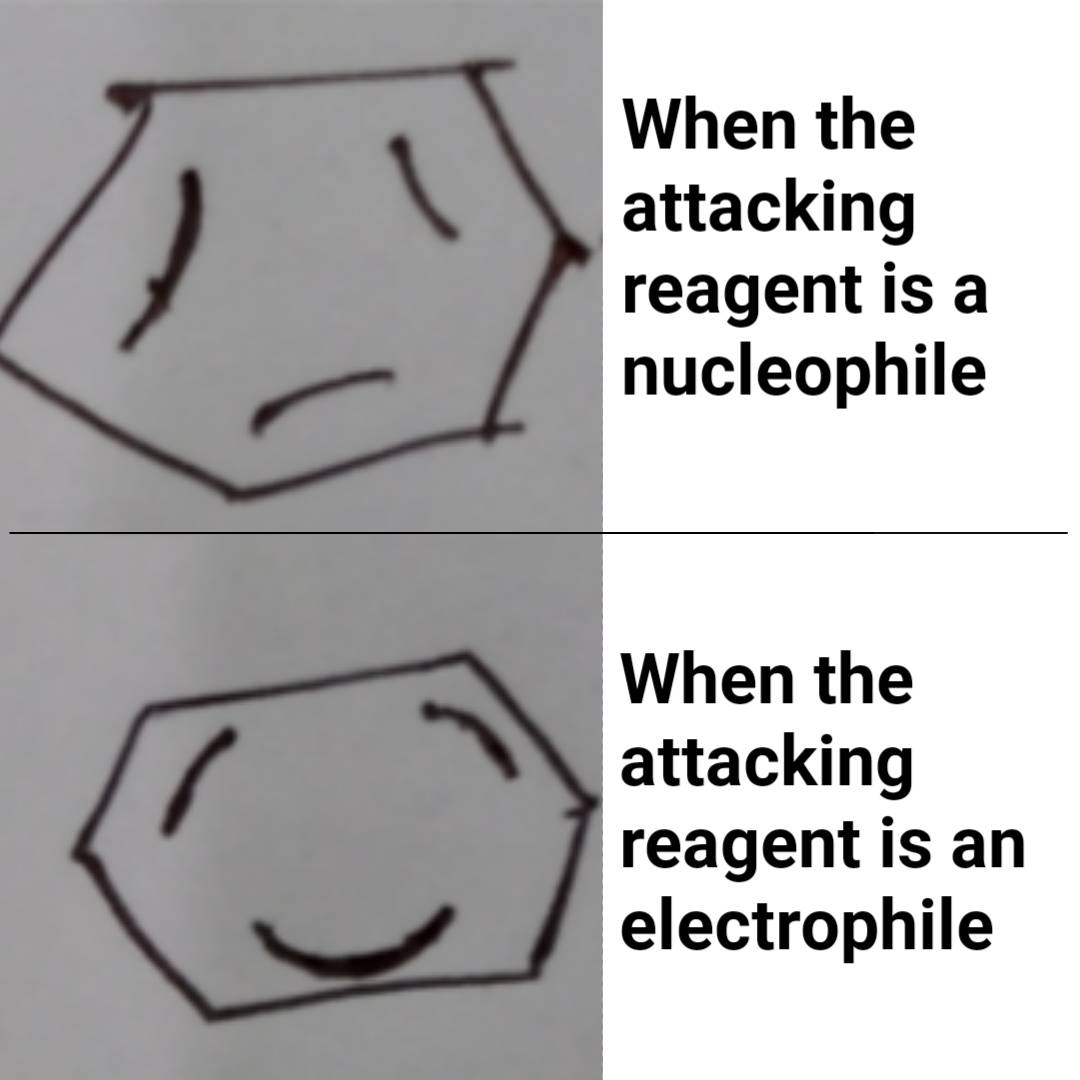
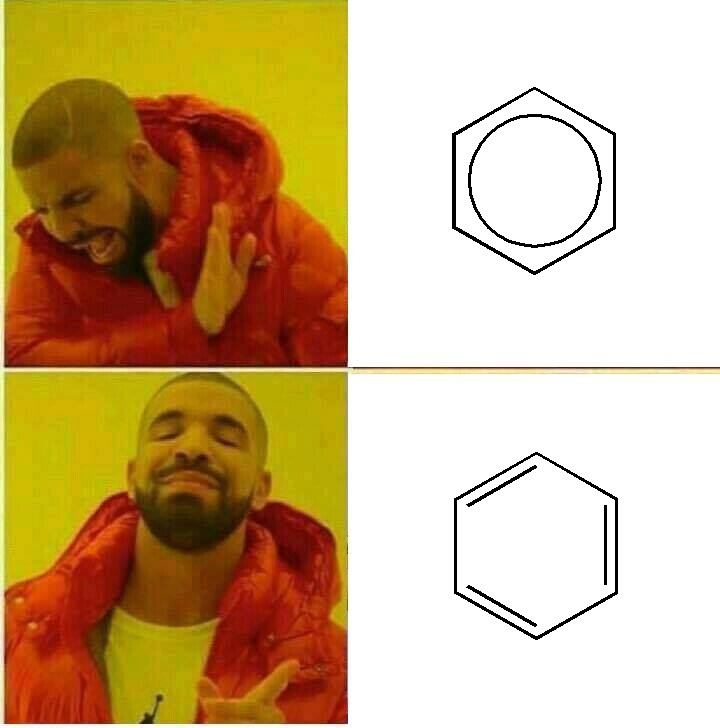
How many scientists does it take to change a light bulb? None, they're still writing the grant proposal.
Ad Breville Barista Express
Turn your kitchen into a hipster café

Buy = You get cool gear + we can finally upgrade our centrifuge from that one model with all the safety issues. 🔒

 Academia
Academia
 Ai
Ai
 Astronomy
Astronomy
 Biology
Biology
 Chemistry
Chemistry
 Climate
Climate
 Conspiracy
Conspiracy
 Earth-science
Earth-science
 Engineering
Engineering
 Evolution
Evolution
 Geology
Geology